Abstract
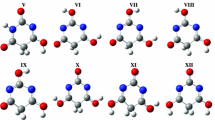
Ab initoand density functional theory (DFT) methods were used to study the tautomers of barbituric acid in the gas phase and in a polar medium. In the gas phase, the tautomers were optimized at the HF/6-31G*, MP2/6-31G*and B3LYP/6-31G*, B3PW91/6-31G*levels of theory. The self-consistent reaction field theory (SCRF) at the HF/6-31G*level of theory has been used to optimize the tautomers in a polar medium. The relative stability of the tautomers was compared in the gaseous and polar mediums. The ability of maximum hardness principle to predict the stable tautomer has been studied. The 13C-NMR chemical shift for carbon atoms in the tautomers was calculated and the results are discussed.
Similar content being viewed by others
References
Maruizumi, T., Hiyama, Y. and Niki, E., Bull. Chem. Soc. Jpn., 53 (1980) 1443.
Zhang, X.-M., Malick, D. and Petersson, G.A., J. Org. Chem., 63 (1998) 5314.
Keeffe, J.R., Kresge, A.J. and Chepp, N.P., J. Am. Chem. Soc., 112 (1990) 4862.
Chiang, Y. and Kresge, A.J., Science, 253 (1991) 395.
Selzer, T. and Rappoport, Z., J. Org. Chem., 61 (1996) 5462.
Jeffrey, G.A., Ghose, S. and Warwicker, J.O., Acta. Cryst., 14 (1961) 881.
Kakkar, R. and Katoch, V., Proc. Ind. Acad. Sci. (Chem. Sci.), 110(6) (1998) 535.
Sanz, J.F., Anguiano, J. and Vilarrasa, J., Comput. Chem., 9 (1988) 784.
Anguiano, J., Sanz, J.F. and Vilarrasa, J., J. Org. Chem., 53 (1988) 3900.
Gould, I.R. and Hillier, I.H., Chem. Phys. Lett., 161 (1989) 185.
Senthilkumar, K. and Kolandaivel, P., J. Mol. Struct. (THEOCHEM), 577(1) (2002) 69.
Lundquist, I, Phys. Rev. Lett., 76 (1996) 102.
Dobson, J.F. and Wang, J., Phys. Rev. Lett., 82 (1999) 2123.
Lein, M., Dobson, J.F. and Gross, E.K.U., J. Comput. Chem., 20 (1999) 12.
Kohn, W., Meir, Y. and Markov, D.E., Phys. Rev. Lett., 80 (1998) 4153.
Dobson, J.F., Vignale, G. and Das, M.P. (eds), Electronic Density Functional Theory: Recent Progress and New Directions, Plenum, New York, NY, 1997.
Kim, K. and Jordan, K.D., Chem. Phys. Lett., 218 (1994) 261.
Chong, D.P., Chem. Phys. Lett., 217 (1994) 539.
Sosa, C., Lee. C., Fitzgerald, G. and Eades, R.A., Chem. Phys. Lett., 211 (1993) 265.
Sim, F., Amant, A.S., Papai, I. And Salahub, D.R., J. Am. Chem. Soc., 114 (1992) 431.
Rubin, Y.V., Morozov, Y., Venkateswarlu, D. and Leszczynski, J., J. Phys. Chem., 102 (1998) 2194.
Katritzky, A.R. and Boulton, A.J., (eds), Advances in Heterocyclic Chemistry, Academic Press, New York, 1976.
Jaikumar, N. and Kolandaivel, P., Int. J. Quant. Chem., 76 (2000) 648.
Senthilkumar, K and Kolandaivel, P., computers and chemistry, 26(3) (2002) 207.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Gill, P.M.W., Johnson, B.G., Robb, M.A., Cheeseman, J.R., Keith, T., Petersson, G.A., Montgomery, J.A., Raghavachari, K., Al-Laham, M.A., Zakrzewski, V.G., Ortiz, J.V., Foresman, J.B., Cioslowski, J., Stefanov, B.B., Nanayakkara, A., Challacombe, M., Peng, C.Y., Ayala, P.Y., Chen, W., Wong, M.W., Andres, J.L., Replogle, E.S., Gomperts, R., Martin, R.L., Fox, D.J., Binkley, J.S., Defrees, D.J., Baker, V., Stewart, J.P., Head-Gordon, M., Gonzalez, C. and Pople, J.A., Gaussian 94, Revision B.2., Gaussian, Inc., Pittsburgh, PA, 1995.
Møller, C. and Plesset, M.S., Phys. Rev., 46 (1934) 618.
Beke, A.D., Phys. Rev., A38 (1988) 3098.
Lee, C., Yang, W. Parr, R.G., Phys. Rev., B37 (1988) 785.
Predew, J.P. and Wang, Y., Phys. Rev., B45 (1992) 244.
Miertus, S., Scrocco, E. and Tomasi, J., Chem. Phys., 55 (1981) 117.
Ditchfield, R., Mol. Phys., 27 (1974) 789.
Wolinski, K., Hinton, J.F. and Fulay, P., J. Am. Chem. Soc., 112 (1990) 8251.
Cotton, F.A. and Feng, X., J. Am. Chem. Soc., 120 (1997) 3387.
Arulmozhiraja, S., Kolandaivel, P. and Ohashi, O., J. Phys. Chem., A103(16) (1999) 3073.
Tsipis, A.C. and Tsipis, C.A., Phys. Chem. Chem. Phys., 1 (1999) 4541.
Civalleri, B., Garrone, E. and Ugliengo, P., J. Mol. Struct. (THEOCHEM), 419 (1997) 227.
Chen, Y. and Roux, E.T., J. Phys. Chem., 96 (1992) 7266.
El-Azhary, A.A. and Suter, H.U., J. Phys. Chem., 100 (1996) 15056.
Yekeler, H., J. Comput. Aid. Mol. Des., 14 (2000) 243.
Soundararajan, S., Trans. Faraday Soc., 54 (1958) 1147.
Kwiatkowski, J.S., Bartlett, R.J. and Person, W.B., J. Am. Chem. Soc., 110 (1988) 2353.
Kolandaivel, P. and Senthilkumar, K. J. Mol. Struct. (THEOCHEM), 535 (2001) 61.
Gorb, L. and Leszezynski, J., J. Am. Chem. Soc., 120 (1998) 5024.
Silverstein, R.M., Bassler, G.C. and Morrill, T.C. (eds), Spectrometric Identification of Organic Compounds, John Wiley and Sons. Inc., New York, NY, 1991.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Senthilkumar, K., Kolandaivel, P. Quantum chemical studies on tautomerism of barbituric acid in gas phase and in solution. J Comput Aided Mol Des 16, 263–272 (2002). https://doi.org/10.1023/A:1020273219651
Issue Date:
DOI: https://doi.org/10.1023/A:1020273219651