Abstract
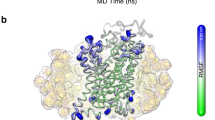
The dopamine transporter (DAT) regulates the action of dopamine by reuptake of the neurotransmitter into presynaptic neurons, and is the main molecular target of amphetamines and cocaine. DAT and the Na+/H+ antiporter (NhaA) are secondary transporter proteins that carry small molecules across a cell membrane against a concentration gradient, using ion gradients as energy source. A 3-dimensional projection map of the E. coli NhaA has confirmed a topology of 12 membrane spanning domains, and was previously used to construct a 3-dimensional NhaA model with 12 trans-membrane α-helices (TMHs). The NhaA model, and site directed mutagenesis data on DAT, were used to construct a detailed 3-dimensional DAT model using interactive molecular graphics and empiric force field calculations. The model proposes a dopamine transport mechanism involving TMHs 1, 3, 4, 5, 7 and 11. Asp79, Tyr252 and Tyr274 were the primary cocaine binding residues. Binding of cocaine or its analogue, (−)-2β-carbomethoxy-3β-(4-fluorophenyl)tropane (CFT), seemed to lock the transporter in an inactive state, and thus inhibit dopamine transport. The present model may be used to design further experimental studies of the molecular structure and mechanisms of DAT and other secondary transporter proteins.
Similar content being viewed by others
References
Reizer, J., Reizer, A. and Saier, M.H., Jr., Biochim. Biophys. Acta, 1197 (1994) 133.
Saier, M.H., Jr., Microbiol Mol Biol Rev, 64 (2000) 354.
Rothman, A., Padan, E. and Schuldiner, S., J. Biol. Chem., 271 (1996) 32288.
Kaback, H.R. and Wu, J., Q. Rev. Biophys., 30 (1997) 333.
Chen, J.G., Liu-Chen, S. and Rudnick, G., J. Biol. Chem., 273 (1998) 12675.
Giros, B., el Mestikawy, S., Godinot, N., Zheng, K., Han, H., Yang-Feng, T. and Caron, M.G., Mol. Pharmacol., 42 (1992) 383.
Edvardsen, O. and Dahl, S.G., Brain Res. Mol. Brain. Res., 27 (1994) 265.
Ravna, A.W. and Edvardsen, O., J. Mol. Graph. Model., 20 (2001) 133.
Ravna, A.W., Sylte, I. and Dahl, S.G., Receptors and Channels, 7 (2001) 319.
Zeng, H., Parthasarathy, R., Rampal, A.L. and Jung, C.Y., Biophys. J., 70 (1996) 14.
Dwyer, D.S., Proteins, 42 (2001) 531.
Uhl, G., Lin, Z., Metzger, T. and Dar, D.E., Methods Enzymol., 296 (1998) 456.
Boja, J.W., Vaughan, R., Patel, A., Shaya, E.K. and Kuhar, M.J., In Boja, J.W., Vaughan, R., Patel, A., Shaya, E.K. and Kuhar, M.J. (Eds), Dopamine receptors and transporters, Marcel Dekker, New York, 1994, 611.
Williams, K.A., Nature, 403 (2000) 112.
Hrynchuk, R.J., Barton, R.J. and Robertson, B.E., Can. J. Chem., 61 (1983) 481.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Gill, W., Johnson, B.G., Robb, M.A., Cheeseman, J.R., Keith, T., Petersson, G.A., Montgomery, J.A., Raghavachari, K., Al-Laham, M.A., Zakrzewski, V.G., Ortiz, J.V., Foresman, J.B., Cioslowski, J., Stefanov, B.B., Nanayakkara, A., Challacombe, M., Peng, C.Y., Ayala, P.Y., Chen, W., Wong, M.W., Andre, J.L., Replogle, E.S., Gomperts, R., Martin, R.L., Fox, D.J., Binkley, J.S., Defrees, D.J., Baker, J., Stewart, J.P., Head-Gordon, M., Gonzales, C. and Pople, J.A., Gaussian 94, 1995, Gaussian Inc: Pittsburg PA.
Ravna, A.W., Schroder, K.E. and Edvardsen, O., Comput. Chem., 23 (1999) 435.
Kristiansen, K., Edvardsen, O. and Dahl, S.G., Med. Chem. Res., 3 (1993) 370.
Charifson, P.S., Hiskey, R.G. and Pedersen, L.G., J. Comput. Chem., 11 (1990) 1181.
Giesecke, J., Acta Crystallogr., B36 (1980) 178.
Ferrin, T.E., Huang, C.C., Jarvis, L.E. and Langridge, R., J. Mol. Graphics, 6 (1988) 13.
Cornell, W.D., Cieplak, P., Bayly, C.I., Gould, I.R., Merz, K.M., Ferguson, D.M., Spellmeyer, D.C., Fox, T., Caldwell, J.W. and Kollman, P.A., J. Am. Chem. Soc., 117 (1995) 5179.
Christensen, I.T. and Jorgensen, F.S., J Biomol Struct Dyn, 15 (1997) 473.
Fanelli, F., Menziani, C., Scheer, A., Cotecchia, S. and De Benedetti, P.G., Methods, 14 (1998) 302.
Sylte, I., Bronowska, A. and Dahl, S.G., Eur. J. Pharmacol., 416 (2001) 33.
Berman, H.M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T.N., Weissig, H., Shindyalov, I.N. and Bourne, P.E., Nucleic Acids Res., 28 (2000) 235.
Lin, Z., Wang, W., Kopajtic, T., Revay, R.S. and Uhl, G.R., Mol. Pharmacol., 56 (1999) 434.
Lin, Z., Itokawa, M. and Uhl, G.R., Faseb. J., 14 (2000) 715.
Kitayama, S., Shimada, S., Xu, H., Markham, L., Donovan, D.M. and Uhl, G.R., Proc. Natl. Acad. Sci. U S A, 89 (1992) 7782.
Kitayama, S., Wang, J.B. and Uhl, G.R., Synapse, 15 (1993) 58.
Kitayama, S., Morita, K., Dohi, T., Wang, J.B., Davis, S.C. and Uhl, G.R., Ann. N. Y. Acad. Sci., 801 (1996) 388.
Barker, E.L., Perlman, M.A., Adkins, E.M., Houlihan, W.J., Pristupa, Z.B., Niznik, H.B. and Blakely, R.D., J. Biol. Chem., 273 (1998) 19459.
Chen, J.G., Sachpatzidis, A. and Rudnick, G., J. Biol. Chem., 272 (1997) 28321.
Mitsuhata, C., Kitayama, S., Morita, K., Vandenbergh, D., Uhl, G.R. and Dohi, T., Brain Res. Mol. Brain Res., 56 (1998) 84.
Sur, C., Betz, H. and Schloss, P., Proc. Natl. Acad. Sci. U S A, 94 (1997) 7639.
Sur, C., Schloss, P. and Betz, H., Biochem. Biophys. Res. Commun., 241 (1997) 68.
Barker, E.L. and Blakely, R.D., Mol. Pharmacol., 50 (1996) 957.
Lee, S.H., Chang, M.Y., Lee, K.H., Park, B.S., Lee, Y.S. and Chin, H.R., Mol. Pharmacol., 57 (2000) 883.
Smicun, Y., Campbell, S.D., Chen, M.A., Gu, H. and Rudnick, G., J. Biol. Chem., 274 (1999) 36058.
Chen, J.G. and Rudnick, G., Proc. Natl. Acad. Sci. U S A, 97 (2000) 1044.
Bairoch, A. and Apweiler, R., Nucleic. Acids. Res., 27 (1999) 49.
Rost, B., Meth. Enzymol., 266 (1996) 525.
Kuroda, T., Shimamoto, T., Mizushima, T. and Tsuchiya, T., J. Bacteriol., 179 (1997) 7600.
Noumi, T., Inoue, H., Sakurai, T., Tsuchiya, T. and Kanazawa, H., J. Biochem. (Tokyo), 121 (1997) 661.
Inoue, H., Noumi, T., Tsuchiya, T. and Kanazawa, H., FEBS Lett., 363 (1995) 264.
Gerchman, Y., Olami, Y., Rimon, A., Taglicht, D., Schuldiner, S. and Padan, E., Proc. Natl. Acad. Sci. U S A, 90 (1993) 1212.
Gerchman, Y., Rimon, A. and Padan, E., J. Biol. Chem., 274 (1999) 24617.
Olami, Y., Rimon, A., Gerchman, Y., Rothman, A. and Padan, E., J. Biol. Chem., 272 (1997) 1761.
Rimon, A., Gerchman, Y., Kariv, Z. and Padan, E., J. Biol. Chem., 273 (1998) 26470.
Carroll, F.I., Gao, Y.G., Rahman, M.A., Abraham, P., Parham, K., Lewin, A.H., Boja, J.W. and Kuhar, M.J., J. Med. Chem., 34 (1991) 2719.
Pearson, W.R. and Lipman, D.J., Proc. Natl. Acad. Sci. USA, 85 (1988) 2444.
Loland, C.J., Norregaard, L. and Gether, U., J. Biol. Chem., 274 (1999) 36928.
Nicholls, A., Sharp, K.A. and Honig, B., Proteins, 11 (1991) 281.
Nicholls, A., GRASP: Graphical Representation and Analysis of Surface Properties, 1992.
Chang, G. and Roth, C.B., Science, 293 (2001) 1793.
Murakami, S., Nakashima, R., Yamashita, E. and Yamaguchi, A., Nature, 419 (2002) 587.
Kawabe, T., Fujihira, E. and Yamaguchi, A., J. Biochem. (Tokyo), 128 (2000) 195.
Hirai, T., Heymann, J.A., Shi, D., Sarker, R., Maloney, P.C. and Subramaniam, S., Nat. Struct. Biol., 9 (2002) 597.
Bennett, E.R. and Kanner, B.I., J. Biol. Chem., 272 (1997) 1203.
Olivares, L., Aragon, C., Gimenez, C. and Zafra, F., J. Biol. Chem., 272 (1997) 1211.
Yu, N., Cao, Y., Mager, S. and Lester, H.A., FEBS Lett., 426 (1998) 174.
Hruz, P.W. and Mueckler, M.M., Biochemistry, 39 (2000) 9367.
Kleinberger-Doron, N. and Kanner, B.I., J. Biol. Chem., 269 (1994) 3063.
Larsen, N.A., Turner, J.M., Stevens, J., Rosser, S.J., Basran, A., Lerner, R.A., Bruce, N.C. and Wilson, I.A., Nat Struct Biol, 9 (2002) 17.
Rasmussen, S.G., Carroll, F.I., Maresch, M.J., Jensen, A.D., Tate, C.G. and Gether, U., J. Biol. Chem., 276 (2001) 4717.
Mortensen, O.V., Kristensen, A.S. and Wiborg, O., J. Neurochem., 79 (2001) 237.
Ferrer, J.V. and Javitch, J.A., Proc. Natl. Acad. Sci. USA, 95 (1998) 9238.
Loland, C.J., Norregaard, L., Litman, T. and Gether, U., Proc. Natl. Acad. Sci. USA, 99 (2002) 1683.
Fischer, J.F. and Cho, A.K., J. Pharmacol. Exp. Ther., 208 (1979) 203.
Liang, N.Y. and Rutledge, C.O., Biochem. Pharmacol., 31 (1982) 983.
Faivre, V., Manivet, P., Callaway, J.C., Airaksinen, M.M., Morimoto, H., Baszkin, A., Launay, J.M. and Rosilio, V., FEBS Lett., 471 (2000) 56.
Chen, N., Ferrer, J.V., Javitch, J.A. and Justice, J.B., Jr., J. Biol. Chem., 275 (2000) 1608.
Reith, M.E., Berfield, J.L., Wang, L.C., Ferrer, J.V. and Javitch, J.A., J. Biol. Chem., 276 (2001) 29012.
Schmid, J.A., Scholze, P., Kudlacek, O., Freissmuth, M., Singer, E.A. and Sitte, H.H., J. Biol. Chem., 276 (2001) 3805.
Kilic, F. and Rudnick, G., Proc. Natl. Acad. Sci. USA, 97 (2000) 3106.
Hastrup, H., Karlin, A. and Javitch, J.A., Proc. Natl. Acad. Sci. USA, 98 (2001) 10055.
Chang, A.S., Starnes, D.M. and Chang, S.M., Biochem. Biophys. Res. Commun., 249 (1998) 416.
Thompson, J.D., Higgins, D.G. and Gibson, T.J., Nucleic Acids Res., 22 (1994) 4673.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ravna, A.W., Sylte, I. & Dahl, S.G. Molecular model of the neural dopamine transporter. J Comput Aided Mol Des 17, 367–382 (2003). https://doi.org/10.1023/A:1026116017725
Issue Date:
DOI: https://doi.org/10.1023/A:1026116017725