Abstract
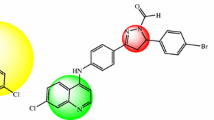
One of the most important pharmacological mechanisms of antimalarial action is the inhibition of the aggregation of hematin into hemozoin. We present a group of new potential antimalarial molecules for which we have performed a DFT study of their stereoelectronic properties. Additionally, the same calculations were carried out for the two putative drug receptors involved in the referred activity, i.e., hematin μ-oxo dimer and hemozoin. A complementarity between the structural and electronic profiles of the planned molecules and the receptors can be observed. A docking study of the new compounds in relation to the two putative receptors is also presented, providing a correlation with the defined electrostatic complementarity.
Similar content being viewed by others
References
Winstanley, P.A., Parasitol. Today, 16 (2000) 146.
Greenwood, B.M., Parasitol. Today, 13 (1997) 90.
Koella, J.C., Parasitol. Today, 14 (1998) 360.
Bray, P.G., Mungthin, M., Ridley, R.G. and Ward, S.A., Mol. Pharm., 54 (1998) 170.
Ginsburg, H. and Krugliak, M., Drug Resistance Updates, 2 (1999)180.
Francis, S.E., Sullivan, D.J., Jr. and Goldberg D.E., Annu. Rev. Microbiol., 51 (1997) 97.
Blauer, G. and Akkawi, M.J., Inorg. Biochem., 66 (1997) 145.
Pagola, S., Stephens, P.W., Bohle D.S., Kosar, A.D. and Madsen S.K., Nature, 404 (2000) 307.
Bohle, D.S., Dinnebier, R.E., Madsen, S.K. and Stephens, P.W., J. Biol. Chem., 272 (1997) 713.
Dorn, A., Stoffel, R., Matile, H., Bubendorf, A. and Ridley, R.G., Nature, 374 (1995) 269.
Fitch, C. D. and Chou, A.C., Antimicrob. Agents Chemother., 41 (1997) 2461.
Zhang, J., Krugliak, M. and Ginsburg, H., Mol. Biochem. Parasitol., 99 (1999) 129.
Vippagunta, S.R., Dorn, A., Ridley, R.G. and Vennerstrom, J.L., Biochim. Biophys. Acta, 1475 (2000) 133.
Moreau, S., Perly, B. and Biguet, J., Biochimie, 64 (1982) 1015.
Moreau, S., Perly, B., Chachaty, C. and Deleuze, C., Biochim. Biophys. Acta, 840 (1985) 107.
Chou, A.C. and Fitch, C.D., J. Clin. Invest., 66 (1980) 856.
Orjih, A.U., Banyal, H.S., Chevli, R. and Fitch, C.D., Science, 214 (1981) 667.
Ginsburg, H. and Demel, R.A., Biochim. Biophys. Acta, 772 (1983) 316.
Hebbel, R.P. and Eaton, J.W., Semin. Hematol., 26 (1989) 136.
Ginsburg, H., Famin, O., Zhang, J. and Krugliak, M., Biochem. Pharmacol., 56 (1998) 1305.
Sugioka, Y. and Suzuki, M., Biochim. Biophys. Acta, 1074 (1991) 19.
Chou, A.C., Chevli, R. and Fitch, C.D., Biochemistry, 19 (1980) 1543.
Egan, T.J., Mavuso, W.W., Ross, D.C. and Marques, H.M., J. Inorg. Biochem., 68 (1997) 137.
Sullivan, D.J., Jr., Gluzman, I.Y., Russell, D.G. and Goldberg, D.E., Proc. Natl. Acad. Sci. USA, 93 (1996) 11865.
Sullivan, D. J., Jr., Matile, H., Ridley R.G. and Goldberg, D.E., J. Biol. Chem., 273 (1998) 31103.
Dorn, A., Vippagunta, S.R., Matile, H., Jaquet, C., Vennerstrom, J. L. and Ridley, R. G., Biochem. Pharmacol., 55 (1998) 727.
Orjih, A.U. and Fitch, C.D., Biochim. Biophys. Acta, 1157 (1993) 270.
Egan, T.J., Ross, D. C. and Adams, P.A., FEBS Lett., 352 (1994) 54.
Ursos, L.M.B., DuBay, K.F. and Roepe, P.D., Mol. Biochem. Parasitol., 112 (2001) 11.
Egan, T.J., Hempelmann, E. and Mavuso, W.W., J. Inorg. Biochem., 73 (1999) 101.
Foley, M. and Tilley, L., Pharmacol. Ther., 79 (1998) 55.
Hawley, S.R., Bray, P.G., Mungthin, M., Atkinson, J.D., O'Neill, P.M. and Ward, S.A., Antimicrob. Agents Chemother., 42 (1998) 682.
Egan, T.J. and Marques, H.M., Coord. Chem. Rev., 190-192 (1999) 493.
Egan, T.J., Exp. Opin. Ther. Patents, 11 (2001) 185.
Ziegler, J., Linck, R. and Wright, D.W., Curr. Med. Chem., 8 (2001) 171.
Winter, R.W., Cornell, K.A., Johnson, L.L., Ignatushenko, M., Hinrichs, D.J. and Riscoe, M.K., Antimicrob. Agents Chemother., 40 (1996) 1408.
Winter, R.W., Ignatushenko, M., Ogundahunsi, O.A.T., Cornell, K.A., Oduola, A.M.J., Hinrichs, D.J. and Riscoe, M.K., Antimicrob. Agents Chemother., 41 (1997) 1449.
Ignatushenko, M., Winter, R.W., Bachinger, H.P., Hinrichs, D.J. and Riscoe, M.K., FEBS Lett., 409 (1997) 67.
Ignatushchenko, M.V., Winter, R.W. and Riscoe, M.K., Am. J. Trop. Med. Hyg., 62 (2000) 77.
Vippagunta, S.R., Dorn, A., Matile, H., Battacharjee, A.K., Karle, J.M., Ellis, W.Y., Ridley, R.G. and Vennerstrom, J.L., J. Med. Chem., 42 (1999) 4630.
De, D., Krogstad, F.M., Byers, L.D. and Krogstad, D.J., J. Med. Chem., 41 (1998) 4918.
Egan, T.J., Hunter, R., Kaschula, C.H., Marques, H.M., Misplon, A. and Walden, J., J. Med. Chem., 43 (2000) 283.
De, D., Krogstad, F.M., Cogswell, F.B. and Krogstad, D.J., Am. J. Trop. Med. Hyg., 55 (1996) 579.
Ridley, R.G., Hofheinz, W., Matile, H., Jaquet, C., Dorn, A.O., Masciadri, R.O., Jolidon, S.O., Richter, W. F.O., Guenzi, A.O., Girometta, M., Urwyler, H., Huber, W., Thaithong, S. and Peters, W., Antimicrob. Agents Chemother., 40 (1996) 1846.
Battacharjee, A.K., J. Mol. Struct. (Theochem.), 529 (2000) 193.
Battacharjee, A.K., J. Mol. Struct. (Theochem.), 549 (2001) 27.
Battacharjee, A.K. and Karle, J.M., J. Med. Chem., 39 (1996) 4622.
Menezes, C.M.S., Sant'Anna, C.M.R., Rodrigues, C.R. and Barreiro, E.J., J. Mol. Struct. (Theochem.), 579 (2002) 31.
Kaschula, C.H., Egan, T.J., Hunter, R., Basilico, N., Parapini, S., Taramelli, D., Pasini, E. and Monti, D., J. Med. Chem., 45 (2002) 3531.
Hawley, S.R., Bray, P.G., O'Neill, P.M., Park, B.K. and Ward, S.A., Biochem. Pharm., 52 (1996) 723
Becke, A.D., J. Chem. Phys.I, 98 (1993) 5648.
Lee, C., Yang, W. and Parr, R., J. Phys. Rev. B, 37, (1998)785.
Hertwig, R.W. and Koch, W., J. Comput. Chem., 16 (1995) 576.
Jones, G., Willet, P. and Glen, R.C., J. Mol. Biol., 245 (1995) 43.
Jones, G., Willet, P., Glen, R.C., Leach, A.R. and Taylor, R., J. Mol. Biol., 267 (1997) 727.
Jones, G., Willet, P., Glen, R.C., Leach, A.R. and Taylor, R., ACS Symp. Ser., 719 (1999) 271.
Nissink, J.W.M., Murray, C., Hartshorn, M., Verdonk, M.L., Cole, J.C. and Taylor, R., Proteins, 49 (2002) 457.
Eldridge, M.D., Murray, C.W., Auton, T.R., Paolini, G.V. and Mee, R.P., J. Comput.-Aided Mol. Des., 11 (1997) 425.
Baxter, C.A., Murray, C.W., Clark, D.E., Westhead, D.R. and Eldridge, M.D., Proteins, 33 (1998) 367.
Bissantz, C., Folkers, G. and Rognan, D., J. Med. Chem., 43 (2000) 4759.
Schneider, G. and Bohm, H., Drug Discov. Today, 7 (2002) 64.
Beck, H.S. and Hardy, J., Proc. Natl. Acad. Sci. USA, 54 (1965) 286
Siegbahn, P., J. Am. Chem. Soc., 120 (1998) 8417.
Forrester, J. and Frisch, Æ, In Exploring Chemistry with Electronic Structure Methods, Gaussian, Inc., Pittsburgh, PA, 1996; pp. 64 and 157.
Fernandes, P.A. and Ramos, M.J., J. Am. Chem. Soc., 125 (2003) 6311.
Brooks, B.R., Bruccoleri, R.E., Olafson, B.D., States, D.J., Swaminathan, S. and Karplus, M., J. Comput. Chem., 4 (1983) 187.
MacKerell, A.D. Jr., Brooks, B., Brooks III, C.L., Nilsson, L., Roux, B., Won, Y. and Karplus, M., CHARMM: The Energy Function and Its Parameterization with an Overview of the Program, in The Encyclopedia of Computational Chemistry, 1, 271, P. v. R. Schleyer et al., Eds., John Wiley & Sons, Chichester, 1998.
Dewar, M.J.S., Zoebisch, E.G., Healy, E.F. and Stewart, J.J.P., J. Am. Chem. Soc., 107 (1985) 3902.
Voityuk, A.A. and Rösch, N., J. Phys. Chem. A, 104(17) (2000) 4089.
Adams, P.A., Berman, P.A.M., Egan, T.J., Marsh, P.J. and Silver, J., J. Inorg. Biochem., 63 (1996) 69.
Bauminger, E.R., Akkawi, M. and Blauer, G., Inorg. Chim. Acta, 286 (1999) 229.
Bohle, D.S., Debrunner, P., Jordan, P.A., Madsen, S.K. and Schulz, C.E., J. Am. Chem. Soc., 120 (1998) 8255.
Adams, P.A., Egan, T.J., Ross, D.C., Silver, J. and Marsh, P.J., Biochem. J., 318 (1996) 25.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G. E., Robb, M.A., Cheeseman, J.R., Zakrzewski, V.G., Montgomery, Jr., J.A., Stratmann, R.E., Burant, J.C., Dapprich, S., Millam, J.M., Daniels, A.D., Kudin, K.N., Strain, M.C., Farkas, O., Tomasi, J., Barone, V., Cossi, M., Cammi, R., Mennucci, B., Pomelli, C., Adamo, C., Clifford, S., Ochterski, J., Petersson, G.A., Ayala, P.Y., Cui, Q., Morokuma, K., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Cioslowski, J., Ortiz, J.V., Baboul, A.G., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Gomperts, R., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Andres, J.L., Gonzalez, C., Head-Gordon, M., Replogle, E.S. and Pople, J.A., Gaussian 98, Revision A.9, Gaussian, Inc., Pittsburgh, PA, 1998.
Portmann, S. and Lüthi, H.P., CHIMIA, 54 (2000) 766.
www.accelrys.com
Leach, A. R. Molecular Modelling: Principles and Applications (2nd Ed.), Prentice Hall, New York, 2001.
Portela, C., Afonso, C. M. M., Pinto, M. and Ramos, M. J., to be published.
Portela, C., Afonso, C. M. M., Pinto, M. and Ramos, M. J., FEBS Lett., 547 (2003) 217.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Portela, C., Afonso, C.M., Pinto, M.M. et al. Computational studies of new potential antimalarial compounds – Stereoelectronic complementarity with the receptor. J Comput Aided Mol Des 17, 583–595 (2003). https://doi.org/10.1023/B:JCAM.0000005754.24588.a0
Issue Date:
DOI: https://doi.org/10.1023/B:JCAM.0000005754.24588.a0