Abstract
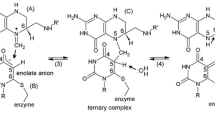
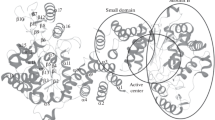
Free energy perturbation calculations have been applied to evaluate the relative free energies of binding of 2′-deoxyuridine-5′-monophosphate (dUMP) and its 2- and/or 4-thio and/or 5-fluoro analogues to the wild-type E. coli thymidylate synthase (ecTS). The results accurately reproduce experimentally measured differences in the free energy of binding of dUMP versus 5-fluoro-dUMP to thymidylate synthase. They indicate that preferred binding of dUMP compared to 5-fluoro-dUMP in the binary complex is equally related to (i) more favorable electrostatic interactions of the dUMP molecule in the enzyme active site, and (ii) its less favorable solvation in the aqueous solution. The relative free energies of binding in the binary complex show moderate and qualitatively indistinguishable discrimination among the studied fluorinated and non-fluorinated 2- and/or 4-thio analogues of dUMP. The binding free energies of monothio analogues of dUMP and 5-fluoro-dUMP correspond quite well with experimentally measured activities of these nucleotides in the thymidylate synthase reaction. On the other hand, the binding free energies of both dithio analogues, 2,4-dithio-dUMP and 2,4-dithio-FdUMP, show lack of such correlation. The latter suggests that very low activities of the dithio analogues of dUMP and 5-fluoro-dUMP may relate more to the covalent reaction of these nucleotides within the ternary complex with TS and 5,10-methylenetetrahydrofolate, than to their pre-covalent binding. We speculate that a lack of substrate activity of 2,4-dithio-dUMP is related to the high aromaticity of its pyrimidine ring that prevents the Michael addition of the active site cysteine thiol to the pyrimidine C6 atom. A stronger affinity of the fluorinated analogues of dUMP to thymidylate synthase, compared to the non-fluorinated congeners, results from the fluorine substituent producing a local strain in the C6 region in the pyrimidine ring, thus sensitizing C6 to the Michael addition of the cysteine thiol.
Similar content being viewed by others
References
Carreras, C.W. and Santi, D.V., Annu. Rev. Biochem., 64 (1995) 721.
Stroud, R.M. and Finer-Moore, J.S., Biochemistry, 42 (2003) 239.
Hartman, K.-R. and Heidelberger, C., J. Biol. Chem., 236 (1958) 3006.
Heidelberger, C., Prog. Nucl. Acid Res. Mol. Biol., 4 (1965) 1.
Smith, G.K., Amyx, H., Boytos, C.M., Duch, D.S., Ferone, R. and Wilson, H.R., Cancer Res., 55 (1995) 6117.
Beale, P. and Clark, S. Tomudex. Clinical development. In: A.L. Jackman (Ed.), Anticancer Drug Development. Humana Press, Inc., Totowa, NJ, 1999. pp. 167-181.
Webber, S., Bartlett, C.A., Boritzki, T.J., Hillard, J.A., Howland, E.F., Johnston, A.L., Kosa, M., Margosiak, S.A., Morse, C.A. and Shetty, B.V., Cancer Chemother. Pharmacol., 37 (1996) 509.
Shih, C. and Thornton, D.E. Preclinical pharmacology studies and the clinical development of a novel multitargeted antifolate, MTA (LY231514). In: A.L. Jackman (Ed.), Anticancer Drug Development. Humana Press, Inc. Totowa, NJ: 1999, pp. 183-201.
Lewis, C.A., Jr. and Dunlap, R.B. Topics in Molecular Pharmacology (Burgen, A.S.V. and Roberts, G.C.K., eds.), Elsevier/North-Holland Biomedical, New York, 1981, pp. 170-219.
Jackman, A.L., Jones, T.R. and Calvert, A.H., in: Muggia, F.M. (Ed.), Experimental and Clinical Progress in Cancer Chemotherapy, Martinus Nijhoff Publishers, Boston, MA, 1985, pp. 155-210.
Eckstein, J.W., Foster, P.G., Finer-Moore, J., Wataya, Y. and Santi, D.V., Biochemistry, 33 (1994) 15086.
Rode, W., Zieliński, Z., Dzik, J.M., Kulikowski, T., Bretner, M., Kierdaszuk, B., Cieśla, J. and Shugar, D., Biochemistry, 29 (1990) 10835.
Dzik, J.M., Bretner, M., Kulikowski, T., Golos, B., Jarmula, A., Poznański, J., Rode,W. and Shugar, D., Biochim. Biophys. Acta, 1293 (1996) 1.
Dzik, J.M., Kulikowski, T., Zieliński, Z., Cieśla, J., Rode, W. and Shugar, D., Biochem. Biophys. Res. Commun., 149 (1987) 1200.
Dzik, J.M., Zieliński, Z., Cieśla, J., Bretner, M., Kulikowski, T., Shugar, D., Bertino, J.R. and Rode, W., Biochem. Biophys. Res. Commun., 195 (1993) 1301.
Kalman, T., Bloch, A., Szekeres, L. and Bardos, T.J., Biochem. Biophys. Res. Commun., 55 (1973) 210.
Bretner, M., Kulikowski, T., Dzik, J.M., Balińska, M., Rode, W. and Shugar, D., J. Med. Chem. 36, (1993) 3611.
Jarmula, A., Anulewicz, R., Leś, A., Cyrański, M.K., Adamowicz, L., Bretner, M., Felczak, K., Kulikowski, T., Krygowski, T.M. and Rode, W., Biochim. Biophys. Acta, 1382 (1998) 277.
Beveridge, D.L. and Di Capua, F.M., Annu. Rev. Biophys. Biophys., 18 (1989) 431.
Kollman, P.A., Chem. Rev. 93 (1993) 2395.
Lamb, M.L. and Jorgensen, W.L., Curr. Opin. Chem. Biol., 1 (1997) 449.
Stout, T.J., Sage, C.R. and Stroud, R.M., Structure, 6 (1998) 839.
Case, D.A., Pearlman, D.A., Caldwell, J.W., Cheatham III, T.E., Ross, W.S., Simmerling, C.L., Darden, T.A., Merz, K.M., Stanton, R.V., Cheng, A.L., Vincent, J.J., Crowley, M., Tsui, V., Radmer, R.J., Duan, Y., Pitera, J., Massova, I., Seibel, G.L., Singh, U.C., Weiner, P.K. and Kollman, P.A., AMBER 6, University of California, San Francisco, CA, 1999.
Cornell, W.D., Cieplak, P., Bayly, C.I., Gould, I.R., Merz, K.M., Ferguson, D.M., Spellmeyer, D.C., Fox, T., Caldwell, J.W. and Kollman, P.A., J. Am. Chem. Soc., 117 (1995) 5179.
Bayly, C.I., Cieplak, P., Cornell, W.D. and Kollman, P.A., J. Phys. Chem., 97 (1993) 10269.
Cieplak, P., Bayly, C.I., Cornell, W.D. and Kollman, P.A., J. Comput. Chem., 16 (1995) 1357.
Jorgensen, W.L., Chandrasekhar, J., Madura, J.D., Impey, R.W. and Klein, M.L., J. Chem. Phys., 79 (1983) 926.
Åqvist, J., J. Phys. Chem., 94 (1990) 8021.
Smith, D.E. and Dang, L.X., J. Chem. Phys., 100 (1994) 3757.
Rastelli, G., Thomas, B., Kollman, P.A. and Santi, D.V., J. Am. Chem. Soc., 117 (1995) 7213.
Berendsen, H.J.C., Postma, J.P.M., Gunsteren, W.F.van, DiNola, A. and Haak, J.R., J. Chem. Phys., 81 (1984) 3684.
Ryckaert, J.P., Ciccotti, G. and Berendsen, H.J.C., J. Comput. Phys., 23 (1977) 327.
van Gunsteren, W.F. and Berendsen, H.J.C., Angew. Chem., Int. Ed. Engl., 29 (1990) 992.
Åqvist, J., Medina, C. and Samuelsson, J.-E., Protein Eng., 7 (1994) 385.
Åqvist, J., Luzhkov, V.B. and Brandsdal, B.O., Acc. Chem. Res., 35 (2002) 358.
Finer-Moore, J., Fauman, E.B., Foster, P.G., Perry, K.M., Santi, D.V. and Stroud, R.M., J. Mol. Biol., 232 (1993) 1101.
Weichsel, A., Montfort, W.R., Cieśla, J. and Maley, F., Proc. Natl. Acad. Sci. U.S.A., 92 (1995) 3493.
Felder, T., Dunlap, R.B., Dix, D. and Spencer, T., Biochim. Biophys. Acta, 1597 (2002) 149.
Santi, D.V., McHenry, C.S., Raines, R.T. and Ivanetich, K.M., Biochemistry, 26 (1987) 8606.
Liu, L. and Santi, D.V., Proc. Natl. Acad. Sci. U.S.A., 90 (1993a) 8604.
Liu, L. and Santi, D.V., Biochemistry, 32 (1993b) 9263.
Morse, R.J., Kawase, S., Santi, D.V., Finer-Moore, J. and Stroud, R.M., Biochemistry, 39 (2000) 1011.
Finer-Moore, J.S., Liu, L., Birdsall, D.L., Brem, R., Apfeld, J., Santi, D.V. and Stroud, R.M., J.Mol. Biol., 276 (1998) 113.
Finer-Moore, J., Montfort, W.R. and Stroud, R.M., Biochemistry, 29 (1990) 6977.
Matthews, D.A., Appelt, K., Oatley, S.J. and Xuong, N.H., J. Mol. Biol., 214 (1990a) 923.
Montfort, W.R., Perry, K.M., Fauman, E.B., Finer-Moore, J.S., Maley, G.F., Hardy, L., Maley, F. and Stroud, R.M., Biochemistry, 29 (1990) 6964.
Matthews, D.A., Villafranca, J.E., Janson, C.A., Smith, W.W., Welsh, K. and Freer, S., J. Mol. Biol., 214 (1990b) 937.
Walsh, A.D., Discuss. Farad. Soc., 2 (1947) 18.
Bent, H.A., Chem. Rev., 61 (1961) 275.
Bent, H.A., J. Inorg. Nucl. Chem., 19 (1961) 43.
Jarmula, A., Cyrański, M.K., Leś, A., Krygowski, T.M. and Rode, W., Polish. J. Chem., 72 (1998) 1958.
Stout, T.J. and Stroud, R.M., Structure, 4 (1996) 67.
Saenger, W. Principles of Nucleic Acid Structure. Springer-Verlag, New York, 1984.
Humphrey, W., Dalke, A. and Schulten, K., J. Mol. Graph., 14 (1996) 33.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jarmuła, A., Cieplak, P., Leś, A. et al. Relative free energies of binding to thymidylate synthase of 2- and/or 4-thio and/or 5-fluoro analogues of dUMP. J Comput Aided Mol Des 17, 699–710 (2003). https://doi.org/10.1023/B:JCAM.0000017377.07094.2e
Issue Date:
DOI: https://doi.org/10.1023/B:JCAM.0000017377.07094.2e