Abstract
Computer-assisted surgery is becoming essential in modern medicine to accurately plan, guide, and perform surgeries. Similarly, Digital Twin technology is expected to be instrumental in the future of surgery, owing to its capacity to virtually replicate patient-specific interventions whilst providing real-time updates to clinicians. This perspective introduces the term Digital Twin-Assisted Surgery and discusses its potential to improve surgical precision and outcome, along with key challenges for successful clinical translation.
Similar content being viewed by others
Introduction
The past few decades have seen radical advancements in both medicine and technology, drastically transforming the surgical field. The most prominent shift was the introduction of minimally invasive surgery (MIS), contributing to a significant reduction in trauma and complications for the patient, hence facilitating recovery periods, reducing hospital stays, and overall healthcare expenses1. Further advancements were realised by the incorporation of robotics and modern imaging technologies in MIS providing a greater degree of precision, better visualisation, and enhanced dexterity during surgery2. Nevertheless, such interventions comprise a series of limitations that have hindered their dominance in the surgical field, mostly associated with high costs and steep learning curves for practitioners to familiarise themselves with such techniques3. Moreover, surgical interventions, irrespective of the technology used, are not always successful, with the occurrence of surgical trauma remaining a major contributor to the mortality and morbidity of patients worldwide4. It is envisaged that surgical outcomes may be improved through the incorporation of innovative digital technologies. The use of such technologies is already gaining traction in the healthcare system, as this continues to shift towards a new era of digital transformation as a result of the recent pandemic. Therefore, implementing digital technologies in future surgery is inevitable5.
The rise of Computer Assisted Surgery (CAS)
The use of digital or computer technologies to plan and perform high precision surgical procedures is now a well-established concept known as Computer-Assisted Surgery (CAS)6. This has been widely implemented in several surgical specialities, such as Computer-Assisted Implant Surgery (CAIS)7, orthopaedic surgery (CAOS)8, image guided surgery or neuronavigation in neurosurgery9, and so forth. CAS also known as ‘digital surgery’10,11,12 can be defined as ‘the use of technology for the enhancement of preoperative planning, surgical performance, therapeutic support, or training, to improve outcomes and reduce harm’11. This predicts the use of cutting-edge digital technology tools such as robotics, Artificial Intelligence (AI) and its subsets, and eXtended Reality (XR: virtual, augmented, mixed) to become major daily contributors in all phases of the surgical lifecycle.
Despite significant advancements of such technologies in surgical settings13,14,15,16,17,18, key challenges and limitations remain to be addressed prior to their widespread clinical use. For example, robotic-assisted surgery is well-known to have a lack of haptic feedback19,20,21, i.e., kinaesthetic (force) and cutaneous (tactile) feedback which are crucial sensory indicators for surgeons during surgery. The robot relies on visual and indirect/resistance feedback rather than true haptic feel. Integrating haptic sensors to robotic-assisted surgery has long been investigated22,23,24,25, and their incorporation in commercial surgical robots is still in its infancy. Recent research development in CAS has been mostly focussed on enhancing the level of assistance since current technologies provide very limited dynamic real-time intraoperative information26. Additionally, CAS technologies fall short in providing predictions to the surgical team27, and therefore, offer a limited contribution to on-the-spot decision-making. For example, when using XR technologies, there is currently a lack of interaction between the physical world i.e., what is happening in the operating theatre, and what is being shown virtually to the surgical team. Such challenges may be overcome by the emerging Digital Twin (DT) technology, that links together synergistically the physical and digital worlds, and is expected to be the new frontier in digital surgery.
DT technology: background and healthcare applications
The history of DT technology dates back to 1970, when NASA employed on-ground simulators of the vehicle used for the Apollo 13 rescue mission. By rapidly adapting and modifying the simulations to closely match conditions as they were evolving in space, the first-ever DT was created. However, the term ‘Digital Twin’ was introduced much later in the 2000s by Prof. Michael Grieves28. Over the years DT technology has evolved greatly, and has been introduced in various industrial sectors, including product development29, manufacturing30,31, infrastructure32, and automotive industries33.
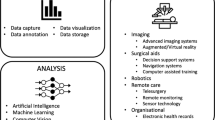
DT technology involves a virtual model of real-world physical objects, processes, or systems based on collected data that is constantly updated and modified in real-time enabling their monitoring, evaluation, prediction, control and optimisation34. Therefore, any changes affecting the physical object are instantaneously mirrored in its virtual counterpart. A DT comprises three main elements: the physical object, the virtual model, and the technologies used to enable communication between the two35, as illustrated in Fig. 1. Modelling and Simulation (MS) technologies, including physical, mechanistic, and statistical simulations, play a leading role in DT construction since without these the virtual element of a DT cannot be realised36,37. AI in DT primarily involves the processing of the ‘big data’ associated with such a technology, which in turn provides insights, predictions, and suggestions, while XR-based technologies enable visualisation and user interaction with the virtual model. The Internet of Things, or in healthcare the Internet of Medical Things (IoT/IoMT)38, comprises network-connected devices, such as sensors, that are crucial for data collection, continuous data exchange, and communication between the twins. Computing power enables large volumes of data to be stored online, thereby widening accessibility even from off-site locations. These are all essential components for data collection and storage, which ultimately provide real-time performance and feedback through interactions between the physical and virtual models34.
A physical object is mirrored virtually with bidirectional real-time interactions (data and information flow). DT technology requires the following core technologies: Artificial Intelligence (AI), the Internet of (Medical) Things (IoT/IoMT), Modelling and Simulation (MS), Cloud Computing (CC), and eXtended Reality (XR). (Icons in figure were designed by Freepik and downloaded with permission from www.flaticon.com.).
DT technology holds great promise in virtually mirroring parts of the human body, from individual cell function to complex tissues and organs. Yet the primary and more ambitious objective is to virtually replicate an entire human body that is patient-specific, thus creating a Digital Human Twin (DHT)39,40. DT technology offers numerous opportunities in healthcare41, for example in medical devices to gain insight into their in vivo functionality and therefore predict future outcomes for the patient, and hospital facilities to manage resources42 and ensure smooth workflow in wards and clinics43.
Digital Twin-Assisted Surgery (DTAS)
Herein, we propose the term “Digital Twin-Assisted Surgery (DTAS)” that integrates DT technology in CAS for assisting perioperative processes to enhance surgical training, planning, precision, safety, and patient care. The novelty of DTAS lies in the real-time virtual model showcasing the interplay between the “objects”, in this case the patient/patient organs being operated on (e.g., kidney, brain, eye) and their physiological parameters (e.g., blood pressure, heart rate, oxygen levels), and the “processes” i.e., surgical intervention (e.g., cutting, suturing, ablation).
DTAS comprises similar elements to other DT applications, as schematically illustrated in Fig. 2. Data is an essential element of a DT irrespective of the target application; therefore, the first stage of DTAS is data acquisition from the physical twin (i.e., tissue, organ, surgical instruments, or intervention) that may be obtained prior to the generation of the virtual DT, or when this is offline, and whilst the DT is active i.e., in real-time. Generally, such data is acquired from medical images (DICOM files from CT, MRI, X-ray, ultrasound), electronic sensors (wearable, physiological, optical, mechanical, and positional), medical devices (glucose and blood pressure monitors), patient health records, molecular and genetic biomarkers, and lab results (blood tests)44,45,46. This may be compiled and stored in electronic databases e.g., using cloud-based storage systems, rendering it accessible to the team responsible for 3D model representation and construction, clinicians, and hospital personnel.
Data is obtained from the physical twin both in real-time and prior to the generation of the digital twin (DT) (offline). This data is analysed using artificial intelligence (AI) and implemented to develop models and simulations, ultimately contributing to the generation of the DT. The DT provides real-time surgical guidance, such as visual and haptic feedback to the surgical team or surgical robot. Extended Reality (XR) may also be used by the surgical team for better visualisation and interaction with the DT. (Icons in figure were designed by Freepik and downloaded with permission from www.flaticon.com.).
The data is analysed via AI and used for the construction of the virtual model46. For example, Deep Learning (DL) methods may be implemented to automatically segment clinical CT or MRI scans, facilitating the reconstruction of the DT 3D geometry47,48. Thorough data analysis is crucial to ensure that: (1) there are no datasets that could introduce bias to the system, (2) all data records are complete and therefore do not promote skewness, (3) there are no inconsistencies that could lead to a weak DT model, and (4) any missing important data fields that could hinder the model’s validity are identified and incorporated. Additional data to run reliable simulations within the clinical timeframes may be based on information from the literature e.g., in vivo and ex vivo studies involving human tissue mechanical characterisation and blood flow dynamics measurements such as cardiac output, while biomechanical, mathematical, and statistical modelling are implemented to emulate physiological conditions. For high-fidelity computational simulations, including finite element analysis (FEA), computational fluid dynamics (CFD), fluid-structure interaction (FSI), fast reduced order models (ROM) calculations, the biomechanical and physical system properties and associated boundary conditions and modelling parameters must also consider factors such as age, gender, heart rate, and organ/tissue pathology which highly influence physiological behaviour45.
Once the virtual model is established, XR technologies may be used by the surgical team for better visualisation and interaction with the model with the additional capability of providing haptic feedback. With real-time data acquisition and analysis, the surgical team is provided with live updates, thus facilitating intraoperative decision-making49. In real-time DTAS, the DT takes the role of a navigator that maps out different surgical routes and possible outcomes to the surgical team—analogous to the Maps applications in our mobile phones where different routes are mapped out to the desired destination that instantaneously update according to one’s location. For instance, in this case, the DT would provide information and predictions on tissue cutting routes to avoid inadvertent damage, and blood flow alterations and blood loss, e.g., when a vessel is clamped or an organ is partially severed50,51. Preoperatively, DTAS allows the surgical team to virtually attempt new techniques or explore different access points and routes prior to the actual intervention on the patient. Postoperatively, DT could facilitate the generation of patient- and case- specific documentation which can contribute to the development of a virtual surgery database45. Given the predictive nature of such technology, DT may also be used for long-term treatment of patients post-surgery52. This may involve abnormality predictions and early disease detection allowing for timely changes in medication and surgical reintervention plans. Ultimately, DTAS shall contribute to enhanced surgical accuracy, and therefore, minimal complications, and a reduction in recovery time for the patient.
Applications of DTAS in various surgical specialities
The concept of DT technology to inform surgical procedures has already been proposed in various specialities53. Table 1 presents some of the recent work published in this area, however given the infancy of DT in surgery, most investigations involve early components of a DT, for example, material constitutive models or specific algorithms towards the generation of a functional DT.
Apart from research investigations, review papers have been published proposing DT frameworks for specific organs, such as the brain54, and surgical procedures55,56,57, highlighting the benefits of DT not just as a surgical assistant, but also as a predictive tool for pharmacological interventions, and for better understanding of organ function (healthy) and dysfunction (pathologies). Moreover, DT’s potential for providing both haptic and visual feedback has also shown great promise in mirroring procedures where this is lacking, such as MIS and robotic surgery58, and is anticipated to facilitate the training of such interventions by providing surgeons with sufficient motor skills to control a surgical robot58,59.
For instance, in orthopaedics DiGioia and Jaramaz60 introduced the concept of “closing the loop” which meant that CAS would not only contribute to intraoperative adjustments but also postoperative monitoring. DT and DTAS will further offer continuous feedback through real-time data from implantable sensors and biometric devices on recovery and complications. Data that will inform postoperative care, adjustment of rehabilitation plans, and refinement of future interventions linking surgical planning, execution and outcome. “Closing the loop” will enable real-time data-driven adjustments, allowing for more personalised treatment strategies and the better care of patients.
Moreover, DTAS also holds immense promise for improving outcomes in complex oncological surgeries and organ-sparing techniques. For example, this technology can be explored in colorectal surgery, hepatopancreatobiliary (HPB) surgery, and urological surgeries such as kidney and bladder cancer surgeries. In colorectal surgeries, the DT can integrate imaging data from CT and MRI to create a virtual model of the colon and surrounding tissues, mapping the exact location of tumours and critical structures like blood vessels61. Surgeons can then use this to plan the optimal resection strategy, minimising damage to healthy tissue. This same approach can be utilised in HPB surgery. Here, surgeons can simulate different resection strategies to maximise tumour removal while preserving liver function. In addition, DTAS can predict how much functional liver tissue will remain after resection, helping avoid post-hepatectomy liver failure. This can also be implemented in kidney-sparing surgeries, such as partial nephrectomies by creating a detailed 3D model of the kidney, tumour, and vasculature. Surgeons can simulate the best cutting plane, ensuring they remove the tumour with clear margins while preserving as much of the healthy kidney as possible. Real-time updates of imaging data62,63 can help to guide ablation procedures such as radiofrequency or cryoablation by showing precise tumour boundaries and thermal damage areas, preventing injury to nearby organs while also ensuring complete ablation of cancerous tissue. Incorporating DTAS into surgery could significantly improve patient outcomes, reduce surgery times, minimise risks, and allow for more personalised and evolving treatment approaches61 with the implementation of new ergonomic paradigms enhancing surgical performance64.
Potential in surgical education and training
DT technology is poised to revolutionise surgical teaching and training, particularly through the integration of advanced robotic-assisted devices that enable multiple operators to collaborate effectively in confined surgical fields18. Traditionally, surgical training has been based on a ‘see one, do one, teach one’ approach coupled with countless repetitions65. Considerable changes have already been implemented through the introduction of AI and XR technologies66,67. However, DT has the potential to take the simulation technologies a step further. By constructing a surgical DT, a repository of surgical knowledge may be generated (e.g., through data from previous surgical performances) that could contribute to the standardisation of certain surgical procedures whilst also making this information accessible to the wider surgical community including experts from across the globe5,55.
DT in surgery allows clinicians to virtually practice complex surgical procedures in a safe, controlled, and realistic environment53,55. This could benefit surgeons irrespective of their level of expertise. DT also provides a platform for surgeons to experiment on surgical cases that are essentially impossible to work on in real-life situations, for example in pregnancy and foetal surgery.
In the same way that current image guided surgery informs and guides the surgeon anatomically, DT will be able to provide additional feedback to the surgeon but will have the benefit of real-time updated information so as to make surgery more precise rather than less precise as the surgery progresses and the anatomy changes. This will make high risk procedures much safer and provide the blueprint or ‘missing link’ for more complex robotic surgery.
In light of this, the introduction of such modern technologies calls for radical changes in the way the surgical training curriculum is devised. As highlighted by the Royal College of Surgeons (England) Future of Surgery report5, surgical training must strive to keep on evolving with the advances in modern technology and take on a multi-disciplinary approach by incorporating knowledge of computing, engineering, and data literacy. However, this does not stop with novice surgical trainees, experienced surgeons must also keep up with new, emerging technologies and surgical techniques.
Potential in telesurgery
The rapid advancements in network communication, such as the latest 5 G and 6 G communication technologies, have been fundamental towards the development of functional DTs, given how much this technology relies on rapid, real-time communication. The use of 5 G communication has also made significant improvements in the field of telesurgery, primarily in minimising latency68,69. Despite this, the translation of telesurgery on humans still faces significant challenges, mostly associated with communication networks such as latency and instability, especially in remote, underdeveloped countries70,71. The implementation of a DT framework comprising AI-based intelligent systems could address such limitations owing to its ability to automatically update based on the virtual model simulations and physical sensor observations, rather than completely relying on the clinicians at the surgical site and those assisting remotely, therefore requiring less data interchange over the network69,70,72.
Challenges to clinical translation
While DT has the potential to offer significant benefits and improvements in surgery owing to its non-invasive, controllable, and repeatable nature73, there still exist key challenges that must be overcome prior to successful clinical translation.
Technical challenges of DTAS
Data related challenges
Issues concerning the translation of DT technology in the medical field, including surgery, are predominantly data related. Contrary to the industrial sector, where DTs mirror non-living objects such as machines, DTs in surgery would mirror complex surgical interventions based on human data, from physiological parameters to complex organ and tissue conditions, to surgical robots and instruments. Such data is collected from a wide range of sources, and in most cases, this is often sparse, noisy, or captured in an unstructured manner, for example, health records that are not digitised, thus adding to the complexity of data acquisition, storage, and analysis40,74. Therefore, reverse-engineering would have to be employed to obtain the appropriate data for the construction of an accurate and reliable DT model75. One way to facilitate this is by creating data-sharing platforms between institutions76, for example from clinics and hospitals, to collect and exchange patient health records, anonymising them when necessary. There is also a need for verification, validation, and uncertainty quantification of the DT model prior to implementing it for clinical decisions and predictions40,74,75, in addition to frequent model recalibrations to match the physical twin based on age, geographical location, gender, and race, to ensure precision and an unbiased treatment37,74.
Advanced sensor technology challenges
Achieving a fully-functional surgical DT, also calls for the development of advanced sensor technologies, including sensors to monitor the surgical process (e.g., tool-tissue interactions)77 which would be useful in providing haptic feedback, and wearable sensors to obtain specific patient health information78. For example, this would involve research on the development of novel materials and the incorporation of microelectromechanical systems (MEMS) for the fabrication of soft, flexible sensors25,79, research in nanotechnology to develop fast response miniature sensors, and advances in microfluidic-based sensor technologies80.
Infrastructure and resources challenges
In a real-time DT, considering that data is continuously fed into the system as the DT model actively updates, a large amount of data is generated that needs to be effectively analysed and stored, requiring state-of-the-art computational power and infrastructure that supports such ‘big data’81. Moreover, to construct and maintain a surgical DT, the input of experts from diverse backgrounds, including surgeons and clinicians, engineers, and data analysts, is required. Thus, this entire process can be time-consuming, and energy- and resource- intensive, which might prove costly and challenging when scaling-up to widespread use in healthcare systems, especially in the case of a real-time DT where computational and network speed are crucial74. Therefore, the right support from companies investing in this technology and the computational framework behind it is required, as well as support from governments, regulatory bodies, and insurance companies to exploit the full benefits of a surgical DT. Ultimately, the goal of DTAS is twofold, to: (1) improve patients’ outcome, and (2) enhance surgical precision through personalised planning (assist the surgical team). Therefore, it is essential to take this into account when developing surgical DTs and ensure that they are implemented where they would drastically contribute to this goal.
Ethical considerations
Data-driven approaches such as surgical DTs, raise a lot of ethical questions82, even more so with DHTs39,83. This is mostly due to the acquisition of copious amounts of personal and sensitive data, that could threaten the patient’s privacy if not handled securely and in an ethical manner. Thus, it is imperative that patients are always provided with informed consent and full transparency, i.e., why is the data being collected, and how will it be managed and shared12, even if the DT will mirror parts of the human body, e.g., organ DTs. This is especially important when real-time data is acquired and fed to the DT during surgery while the patient is unconscious. Following consensual data acquisition, robust cybersecurity measures should be in place to prevent data breaches from cloud-based storage systems, and the misuse of data84. In addition to this, in order to enforce privacy and increase patient trust, sensitive patient data must also be encrypted and equipped with access restrictions40. From the patient’s perspective, the introduction of DTs might evoke the fear that the surgeons are being replaced by machines and computerised models37. Thus, all parties involved in the development of DTs must understand that this technology should by no means replace the surgeon and their expertise but rather assist the surgical team in providing better care for the patient, hence the introduction of the term DTAS. In medicine, the importance of doctor-patient confidentiality is well-established, and this must also be considered in the development of surgical DTs.
Regulatory and legal challenges
Legal frameworks are already in place regarding the collection of patient health data. In the UK this is achieved according to the Data Protection Act of 2018, which implies that there should always be a valid lawful basis as to why data is collected, ultimately to safeguard patient’s privacy. However, similar to the EU’s General Data Protection Regulation (GDPR) and the US’ Health Insurance Portability and Accountability (HIPAA), questions have been raised whether this is enough to keep up with the data-rich, digital transformation85.
Moreover, given that a surgical DT is essentially a medical device, regulatory compliance is key. However, given the infancy of such a technology in the medical field, currently there are limited regulations in place that enforce legal liability with the implementation of DT in surgeries, or rather DT in healthcare as a whole12. The novelty of digital technologies in this sector spurs several uncertainties for regulatory bodies, as well as the need for substantial resources to evaluate the associated risks, and thus, regulatory clearances are typically granted depending on the application. Some of the few existing regulation clearances include the approval granted in September 2022 for AI-driven clinical trials to Unlearn.AI by the European Medicines Association (EMA) (www.unlearn.ai), and the recent clearance by the US Food and Drug Administration (FDA) in March 2024 to inHEART for their AI-driven software to create 3D cardiac models (www.inheartmedical.com). Despite these advancements, there is still a long way to go, therefore it is crucial for major regulatory bodies (the EMA, FDA, and the Medicines and Healthcare products Regulatory Agency (MHRA)) to be involved in DT projects to witness first-hand the DT development, which in turn could facilitate the regulatory pathway. This is already being exploited in major DT projects such as the Living Heart project by Dassault Systèmes where the FDA has been a major collaborator since 2014 (www.3ds.com/heart).
Thus, moving forward, as the DT technology becomes increasingly integrated within the healthcare system, and indeed the whole surgical lifecycle, it is envisaged that ethical and legal issues concerning the use of DTs in clinical settings are properly addressed and standardised37 to reach the full potential of such an innovative and exciting technology. Whereas cybersecurity measures are of the utmost consideration, the chances of hacking while performing surgery-remote interface with DTAS or manipulating patient data are unknown. Consequently, these will raise concerns for the most robust security protocols and real-time monitoring systems to avoid such cyberattacks during surgery84.
Surgeon acceptance
One of the substantial obstacles to overcome in implementing DTAS is acceptance by surgeons. The medical field usually has some resistance to new technologies, which one could witness with the introduction of robotic-assisted surgeries and computer-assisted systems86. Many times, surgeons are more comfortable with traditional methods and may show a little reluctance to be on systems that they perceive as complex, time-consuming, or hard to master. This is particularly true in high-stakes surgeries, where trust in technology can be difficult to establish87. For DTAS to gain broader acceptance, surgeon training and peer advocacy will be crucial. Demonstrating the complementary role of DTAS in improving patient outcomes and enhancing surgical precision rather than replacing the surgeon’s skills will help overcome these barriers. Gradual exposure to the technology, hands-on training, peer-led demonstrations, and patient-centred co-creation will increase surgeon confidence and help enhance overall acceptance of DT-based systems88,89.
Conclusions
Overall, DT technology in surgery has the potential to: (1) assist the surgical team in all stages of surgery for enhanced precision, safety, and patient care, (2) democratise surgery through improved telesurgery, and (3) provide advanced surgical training practices to novice and experienced practitioners alike. Despite these benefits, due to the infancy of DT in this sector, several technical, ethical, and regulatory challenges remain to be addressed prior to its successful clinical translation. Nonetheless, we envisage that in the forthcoming decade through collaborative inter- and multi- disciplinary academic efforts and driven by the substantial investment by major industrial players in robotic as well as DT and AI technologies, DTAS will become instrumental as we progress further into the era of digital surgery.
References
Cuschieri, A. Minimal access surgery and the future of interventional laparoscopy. Am. J. Surg. 161, 404–407 (1991).
Mack, M. J. Minimally Invasive and Robotic Surgery. JAMA 285, 568–572 (2001).
Fuchs, K. Minimally invasive surgery. Endoscopy 34, 154–159 (2002).
Dobson, G. P. Trauma of major surgery: A global problem that is not going away. Int. J. Surg. 81, 47–54 (2020).
Royal College of Surgeons of England. Future of Surgery. (RCS England, London, 2018).
Giménez, M. et al. Definitions of Computer-Assisted Surgery and Intervention, Image-Guided Surgery and Intervention, Hybrid Operating Room, and Guidance Systems: Strasbourg International Consensus Study. Ann. Surg. Open 1, e021 (2020).
D’haese, J., Ackhurst, J., Wismeijer, D., De Bruyn, H. & Tahmaseb, A. Current state of the art of computer-guided implant surgery. Periodontology 2000 73, 121–133 (2017).
Picard, F., Deakin, A. H., Riches, P. E., Deep, K. & Baines, J. Computer assisted orthopaedic surgery: Past, present and future. Med. Eng. Phys. 72, 55–65 (2019).
Grunert, P., Darabi, K., Espinosa, J. & Filippi, R. Computer-aided navigation in neurosurgery. Neurosurgical Rev. 26, 73–99 (2003).
Fuerst, B., Fer, D. M., Herrmann, D. & Kilroy, P. G. in Digital Surgery (ed Sam Atallah) 11-23 (Springer International Publishing, 2021).
Lam, K. et al. A Delphi consensus statement for digital surgery. npj Digital Med. 5, 100 (2022).
Raza, M. M., Venkatesh, K. P., Diao, J. A. & Kvedar, J. C. Defining digital surgery for the future. npj Digital Med. 5, 155 (2022).
Hashimoto, D. A., Rosman, G., Rus, D. & Meireles, O. R. Artificial Intelligence in Surgery: Promises and Perils. Annal. Surg. 268 (2018).
Birkhoff, D. C., van Dalen, A. & Schijven, M. P. A Review on the Current Applications of Artificial Intelligence in the Operating Room. Surg. Innov. 28, 611–619 (2021).
Gupta, A. et al. Artificial intelligence: A new tool in surgeon’s hand. J. Educ. Health Promot 11, 93 (2022).
Zhang, J., Lu, V. & Khanduja, V. The impact of extended reality on surgery: a scoping review. Int. Orthop. 47, 611–621 (2023).
Varghese, C., Harrison, E. M., O’Grady, G. & Topol, E. J. Artificial intelligence in surgery. Nat. Med. 30, 1257–1268 (2024).
Dupont, P. E. et al. A decade retrospective of medical robotics research from 2010 to 2020. Sci. Robot. 6, eabi8017 (2021).
Okamura, A. M. Haptic feedback in robot-assisted minimally invasive surgery. Curr. Opin. Urol. 19, 102–107 (2009).
Enayati, N., De Momi, E. & Ferrigno, G. Haptics in robot-assisted surgery: Challenges and benefits. IEEE Rev. Biomed. Eng. 9, 49–65 (2016).
Dagnino, G. & Kundrat, D. Robot-assistive minimally invasive surgery: trends and future directions. Int. J. Intellig. Robotics Appl. (2024). https://doi.org/10.1007/s41315-024-00341-2.
King, C. H. et al. Tactile Feedback Induces Reduced Grasping Force in Robot-Assisted Surgery. IEEE Trans. Haptics 2, 103–110 (2009).
Kim, U., Lee, D. H., Yoon, W. J., Hannaford, B. & Choi, H. R. Force Sensor Integrated Surgical Forceps for Minimally Invasive Robotic Surgery. IEEE Trans. Robot. 31, 1214–1224 (2015).
Abiri, A. et al. Multi-Modal Haptic Feedback for Grip Force Reduction in Robotic Surgery. Sci. Rep. 9, 5016 (2019).
Hou, C. et al. A Highly Integrated 3D MEMS Force Sensing Module With Variable Sensitivity for Robotic-Assisted Minimally Invasive Surgery. Adv. Funct. Mater. 33, 2302812 (2023).
Zaffino, P., Moccia, S., De Momi, E. & Spadea, M. F. A Review on Advances in Intra-operative Imaging for Surgery and Therapy: Imagining the Operating Room of the Future. Ann. Biomed. Eng. 48, 2171–2191 (2020).
Haidegger, T., Speidel, S., Stoyanov, D. & Satava, R. M. Robot-Assisted Minimally Invasive Surgery—Surgical Robotics in the Data Age. Proc. IEEE 110, 835–846 (2022).
Grieves, M. W. Product lifecycle management: the new paradigm for enterprises. Int. J. Prod. Dev. 2, 71–84 (2005).
Tao, F. et al. Digital twin-driven product design framework. Int. J. Prod. Res. 57, 3935–3953 (2019).
Walker, C. et al. in 2022 8th International Conference on Nanomanufacturing & 4th AET Symposium on ACSM and Digital Manufacturing (Nanoman-AETS). 1-6.
Luo, X., Liu, Q., Madathil, A. P. & Xie, W. Predictive digital twin-driven dynamic error control for slow-tool-servo ultraprecision diamond turning. CIRP Annals (2024). https://doi.org/10.1016/j.cirp.2024.04.080.
Ketzler, B. et al. Digital twins for cities: A state of the art review. Built Environ. 46, 547–573 (2020).
Piromalis, D. & Kantaros, A. Digital Twins in the Automotive Industry: The Road toward Physical-Digital Convergence. Appl. Syst. Innov. 5, 65 (2022).
Attaran, M. & Celik, B. G. Digital Twin: Benefits, use cases, challenges, and opportunities. Decis. Analytics J. 6, 100165 (2023).
Grieves, M. W. in The Digital Twin (eds Noel Crespi, Adam T. Drobot, & Roberto Minerva) 97-121 (Springer International Publishing, 2023).
Wang, B. et al. Human Digital Twin in the context of Industry 5.0. Robot. Computer-Integr. Manuf. 85, 102626 (2024).
Corral-Acero, J. et al. The ‘Digital Twin’ to enable the vision of precision cardiology. Eur. Heart J. 41, 4556–4564 (2020).
Huang, C., Wang, J., Wang, S. & Zhang, Y. Internet of medical things: A systematic review. Neurocomputing 557, 126719 (2023).
de Kerckhove, D. The personal digital twin, ethical considerations. Philos. Trans. R. Soc. A: Math., Phys. Eng. Sci. 379, 20200367 (2021).
Cellina, M. et al. Digital Twins: The New Frontier for Personalized Medicine? Appl. Sci. 13, 7940 (2023).
Katsoulakis, E. et al. Digital twins for health: a scoping review. npj Digital Med. 7, 77 (2024).
Karakra, A., Fontanili, F., Lamine, E., Lamothe, J. & Taweel, A. in 2018 IEEE/ACS 15th International Conference on Computer Systems and Applications (AICCSA). 1-6.
Han, Y., Li, Y., Li, Y., Yang, B. & Cao, L. Digital twinning for smart hospital operations: Framework and proof of concept. Technol. Soc. 74, 102317 (2023).
Armeni, P. et al. Digital Twins in Healthcare: Is It the Beginning of a New Era of Evidence-Based Medicine? A Critical Review. J. Personalized Med. 12, 1255 (2022).
Bjelland, Ø., Pedersen, M., Steinert, M. & Bye, R. Intraoperative Data-Based Haptic Feedback for Arthroscopic Partial Meniscectomy Punch Simulation. IEEE Access PP, 1–1 (2022).
Qin, J. & Wu, J. Realizing the Potential of Computer-Assisted Surgery by Embedding Digital Twin Technology. JMIR Med Inf. 10, e35138 (2022).
Altini, N. et al. Liver, kidney and spleen segmentation from CT scans and MRI with deep learning: A survey. Neurocomputing 490, 30–53 (2022).
Rouhollahi, A. et al. CardioVision: A fully automated deep learning package for medical image segmentation and reconstruction generating digital twins for patients with aortic stenosis. Computerized Med. Imaging Graph. 109, 102289 (2023).
Shu, H. et al. Twin-S: a digital twin for skull base surgery. Int J. Comput Assist Radio. Surg. 18, 1077–1084 (2023).
Cowley, J., Luo, X., Stewart, G. D., Shu, W. & Kazakidi, A. A Mathematical Model of Blood Loss during Renal Resection. Fluids 8, 316 (2023).
Cowley, J. et al. Near Real-Time Estimation of Blood Loss and Flow–Pressure Redistribution during Unilateral Nephrectomy. Fluids 9, 214 (2024).
Keller, J. et al. Using Digital Twins to Support Multiple Stages of the Patient Journey. Stud. Health Technol. Inf. 301, 227–232 (2023).
Ahmed, H. & Devoto, L. The Potential of a Digital Twin in Surgery. Surgical Innov. 28, 509–510 (2021).
Xiong, H. et al. The Digital Twin Brain: A Bridge between Biological and Artificial Intelligence. Intell. Comput. 2, 0055 (2023).
Bjelland, Ø. et al. Toward a Digital Twin for Arthroscopic Knee Surgery: A Systematic Review. IEEE Access 10, 45029–45052 (2022).
Chumnanvej, S., Chumnanvej, S. & Tripathi, S. Assessing the benefits of digital twins in neurosurgery: a systematic review. Neurosurgical Rev. 47, 52 (2024).
Albertini, J.-N., Derycke, L., Millon, A. & Soler, R. Digital twin and artificial intelligence technologies for predictive planning of endovascular procedures. Semin. Vasc. Surg. 37, 306–313 (2024).
Hagmann, K. et al. A Digital Twin Approach for Contextual Assistance for Surgeons During Surgical Robotics Training. Front. Robotics AI 8 (2021). https://doi.org/10.3389/frobt.2021.735566.
Cai, X. et al. Implementation of a Virtual Reality Based Digital-Twin Robotic Minimally Invasive Surgery Simulator. Bioengineering 10 (2023).
DiGioia, A. M. & Jaramaz, B. Computer-assisted tools and interventional technologies. Lancet 354, SIV46 (1999).
Shen, M.-d, Chen, S.-b & Ding, X.-d The effectiveness of digital twins in promoting precision health across the entire population: a systematic review. npj Digital Med. 7, 145 (2024).
Zhu, Z., Liu, C. & Xu, X. Visualisation of the Digital Twin data in manufacturing by using Augmented Reality. Procedia CIRP 81, 898–903 (2019).
Uhlemann, T. H. J., Schock, C., Lehmann, C., Freiberger, S. & Steinhilper, R. The Digital Twin: Demonstrating the Potential of Real Time Data Acquisition in Production Systems. Procedia Manuf. 9, 113–120 (2017).
Hamilton, B. C. S. et al. Artificial intelligence based real-time video ergonomic assessment and training improves resident ergonomics. Am. J. Surg. 226, 741–746 (2023).
Ayub, S. M. See one, do one, teach one”: Balancing patient care and surgical training in an emergency trauma department. J. Glob. Health 12, 03051 (2022).
Guerrero, D. T., Asaad, M., Rajesh, A., Hassan, A. & Butler, C. E. Advancing surgical education: the use of artificial intelligence in surgical training. Am. Surg. 89, 49–54 (2023).
Portelli, M., Bianco, S., Bezzina, T. & Abela, J. Virtual reality training compared with apprenticeship training in laparoscopic surgery: a meta-analysis. Ann. R. Coll. Surg. Engl. 102, 672–684 (2020).
Yang, X. et al. Application of 5G technology to conduct tele-surgical robot-assisted laparoscopic radical cystectomy. Int. J. Med. Robot. Computer Assist. Surg. 18, e2412 (2022).
Dohler, M., Saikali, S., Gamal, A., Moschovas, M. C. & Patel, V. The crucial role of 5G, 6G, and fiber in robotic telesurgery. J. Robotic Surg. 19, 4 (2024).
Bonne, S. et al. in 2022 IEEE 18th International Conference on Automation Science and Engineering (CASE). 1325-1332.
Laaki, H., Miche, Y. & Tammi, K. Prototyping a Digital Twin for Real Time Remote Control Over Mobile Networks: Application of Remote Surgery. IEEE Access 7, 20325–20336 (2019).
Haitao, N. in Artificial Intelligence in Medicine and Surgery - An Exploration of Current Trends, Potential Opportunities, and Evolving Threats - Volume 1 (ed P. Stawicki Dr. Stanislaw) Ch. 14 (IntechOpen, 2023).
Sun, T., He, X. & Li, Z. Digital twin in healthcare: Recent updates and challenges. Digit Health 9, 20552076221149651 (2023).
Niederer, S. A., Sacks, M. S., Girolami, M. & Willcox, K. Scaling digital twins from the artisanal to the industrial. Nat. Computational Sci. 1, 313–320 (2021).
National Academies of Sciences Engineering and Medicine. Opportunities and Challenges for Digital Twins in Biomedical Research: Proceedings of a Workshop–in Brief. (The National Academies Press, 2023).
Jensen, J. & Deng, J. in Companion Proceedings of the ACM Web Conference 2023 p. 989–993 (Association for Computing Machinery, Austin, TX, USA, 2023).
Park, J., Seo, B., Jeong, Y. & Park, I. A Review of Recent Advancements in Sensor-Integrated Medical Tools. Adv. Sci. 11, 2307427 (2024).
Ates, H. C. et al. End-to-end design of wearable sensors. Nat. Rev. Mater. 7, 887–907 (2022).
Dai, Y., Wang, J. & Gao, S. Advanced Electronics and Artificial Intelligence: Must-Have Technologies Toward Human Body Digital Twins. Adv. Intell. Syst. 4, 2100263 (2022).
Othman, W. et al. Tactile Sensing for Minimally Invasive Surgery: Conventional Methods and Potential Emerging Tactile Technologies. Front. Robotics AI 8 (2022). https://doi.org/10.3389/frobt.2021.705662.
Coorey, G. et al. The health digital twin to tackle cardiovascular disease—a review of an emerging interdisciplinary field. npj Digital Med. 5, 126 (2022).
Braun, M. Represent me: please! Towards an ethics of digital twins in medicine. J. Med. Ethics 47, 394 (2021).
Huang, P.-h, Kim, K.-h & Schermer, M. Ethical Issues of Digital Twins for Personalized Health Care Service: Preliminary Mapping Study. J. Med Internet Res 24, e33081 (2022).
Coventry, L. & Branley, D. Cybersecurity in healthcare: A narrative review of trends, threats and ways forward. Maturitas 113, 48–52 (2018).
Lam, K., Purkayastha, S. & Kinross, J. M. The Ethical Digital Surgeon. J. Med Internet Res 23, e25849 (2021).
Picard, F., Clarke, J., Deep, K. & Gregori, A. Computer Assisted Knee Replacement Surgery: Is the Movement Mainstream? Orthopedic & Muscular System 03 (2014). https://doi.org/10.4172/2161-0533.1000153.
BenMessaoud, C., Kharrazi, H. & MacDorman, K. F. Facilitators and Barriers to Adopting Robotic-Assisted Surgery: Contextualizing the Unified Theory of Acceptance and Use of Technology. PLOS ONE 6, e16395 (2011).
Buschemeyer, W. C., Cunningham, D. K. & Edwards, M. J. Surgical training and implementation of emerging surgical technologies. Am. J. Surg. 190, 166–172 (2005).
Nickel, G. C., Wang, S., Kwong, J. C. C. & Kvedar, J. C. The case for inclusive co-creation in digital health innovation. npj Digital Med. 7, 251 (2024).
Aubert, K. et al. Development of Digital Twins to Optimize Trauma Surgery and Postoperative Management. A Case Study Focusing on Tibial Plateau Fracture. Front. Bioeng. Biotechnol. 9 (2021). https://doi.org/10.3389/fbioe.2021.722275.
Ahmadian, H. et al. A digital twin for simulating the vertebroplasty procedure and its impact on mechanical stability of vertebra in cancer patients. Int. J. Numer. Methods Biomed. Eng. 38, e3600 (2022).
Shi, Y. et al. Synergistic Digital Twin and Holographic Augmented-Reality-Guided Percutaneous Puncture of Respiratory Liver Tumor. IEEE Trans. Hum.-Mach. Syst. 52, 1364–1374 (2022).
Acknowledgements
The authors would like to acknowledge the financial support of the UK Research and Innovation (UKRI) Engineering and Physical Sciences Research Council (EPSRC, EP/X033686/1, EP/W004860/1, EP/T024844/1). The funder played no role in the conceptualisation and writing of this manuscript. GDS is supported by The Mark Foundation for Cancer Research (RG95043), the Cancer Research UK Cambridge Centre (C9685/A25177 and CTRQQR-2021\100012) and NIHR Cambridge Biomedical Research Centre (NIHR203312). The views expressed are those of the author and not necessarily those of the NIHR or the Department of Health and Social Care.
Author information
Authors and Affiliations
Contributions
L.A.: Conceptualisation, Visualisation, Writing—original draft, Writing—review and editing. J.K.: Writing—review and editing. X.L., A.K.: Conceptualisation, Writing—review and editing, Funding acquisition. P.C., F. P., K.O.N., S.A.T, and G.D.S: Writing—review and editing, Funding acquisition. W.S.: Conceptualisation, Visualisation, Writing—review and editing, Funding acquisition, Supervision. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
G.D.S. has received educational grants from Pfizer, AstraZeneca, and Intuitive. Surgical consultancy fees from Pfizer, Merck, EUSA Pharma, and CMR Surgical; travel expenses from Pfizer; and speaker fees from Pfizer. G.D.S. is Clinical lead (urology) National Kidney Cancer Audit and Topic Advisor for the NICE kidney cancer guideline. The remaining authors declare no financial or non-financial competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asciak, L., Kyeremeh, J., Luo, X. et al. Digital twin assisted surgery, concept, opportunities, and challenges. npj Digit. Med. 8, 32 (2025). https://doi.org/10.1038/s41746-024-01413-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-024-01413-0