Abstract
Digital therapeutics (DTx) are software-driven solutions for prevention, treatment, and management of medical conditions. Despite a pro-DTx momentum in China, global DTx trial assessments overlooked the country. We identified 756 DTx trials in China and analyzed their characteristics and quality parameters. Over 70% were funded by governments, hospitals, and universities, with tertiary hospitals in eastern China leading most trials. 44.8% used automated DTx, with 39.2% DTx-guided. Most trials focused on management (52.5%) and treatment (38.1%), with few on prevention (9.4%). Mental, behavioral, or neurodevelopmental disorders represented the leading condition category of focus. Recent declines in median sample size, median duration, and mean number of sites were noted. Only 18% of trials were at low overall risk of bias. While recognizing the rapid development of DTx trials in China, we call for better trial design and methodological rigor, prioritization of preventive and primary care, wider condition category scope, and higher inclusivity.
Similar content being viewed by others
Introduction
Digital therapeutics (DTx) are software-driven evidence-based health interventions with positive therapeutic impact on certain medical conditions1,2. By facilitating evidence-based practices through information technologies that can be fully automated for patient self-care or under the support of healthcare professionals3, DTx can significantly improve access to quality health services4. DTx also offer novel approaches for medical conditions where traditional healthcare services and medications are unavailable or ineffective5, and can be much more cost-effective than conventional treatment6,7.
China has been actively promoting digital health technology and services, embedded in grand national initiatives such as “Healthy China”, “Digital China”, and national five-year plans8. Local governments have also introduced measures to foster a research and regulatory environment conducive to the development and application of DTx9,10,11. For these efforts to be effective, similar to other national regulations, DTx require government authorization (either clearance or approval, depending on the risk posed to patients) before commercialization12, which needs to be informed by high-quality clinical trials13.
Public healthcare institutions are the dominant providers of health services in China. However, their revenue primarily comes from service charges reimbursed partially by social health insurance, which includes mandatory health insurance for formal employees and voluntary health insurance heavily subsidized by general government revenue for residents (i.e. those outside formal employment)14. Provider payment is predominantly fee-for-service for ambulatory care and case-based for inpatient care. Demonstrated effectiveness of DTx in disease prevention and management at home may reduce the need for extensive hospital stays. Published Chinese expert consensuses on DTx have consistently highlighted a lack of standardization in product and trial design15,16,17,18.
Recent studies systematically reviewed the characteristics and quality of DTx trials registered in US- and Europe-based registries and agency websites, each using different selection criteria (Supplementary Table 1). Kumar et al. analyzed 68 published clinical studies of 20 Food and Drug Administration (FDA)-authorized prescription DTx available in the US, using highly focused search terms (i.e. brand names, and FDA product codes) on Clinicaltrial.gov, PubMed, the Digital Therapeutic Alliance, and manufactures’ websites19. They assessed trial quality parameters and patient demographics and concluded that prescription DTx trials often lack rigor and inclusivity. Also using focused search terms (i.e. “DTx”, “digital therapeutic”, and “digital intervention”), Yao et al. found 280 DTx trials and identified three types of DTx, namely, disease treatment (50.4%), disease management (42.9%) and health function improvement (6.8%). The study segmented DTx trial development into the embryonic stage (2002–2016), the exploration stage (2017–2020), and the rapid development stage (2020 onwards)20. It also highlighted a lack of a standardized system for clinical design.
Miao et al. used a broader range of search terms and identified 449 DTx trials on FDA-regulated device products21. This study highlighted issues with inclusivity (e.g. those with comorbidities, pregnant women, children, or those not fluent in English) that affect their real-world applicability. It reported a primary focus on nervous system diseases and nutritional and metabolic diseases. Masanneck and Stern further included premarket trials and identified 5889 DTx-related trials. They noted a concentration on mental health DTx, observed an increase in the mean sample size, and emphasized the need for more robust trial designs22.
Recent studies on DTx trials overlooked the Chinese Clinical Trial Registry (ChiCTR), where most clinical trials in China are registered. Considering the pro-DTx momentum in China, such omission means neglection of both a potentially powerful source of DTx innovation and of how well DTx trials address the health needs of the Chinese 1.4 billion population. To fill such important knowledge gaps, we conducted an overview of the DTx trial landscape in China, by identifying and analyzing DTx trials registered on ChiCTR or ClinicalTrials.gov. Building on previous literature about DTx trials globally and considering the characteristics of DTx in China, we examined the trials in terms of key features of interventions, inclusivity, and quality of the Chinese DTx trials.
Results
General characteristics
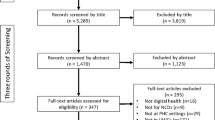
Our systematic search of ChiCTR and Clinicaltrial.gov identified a total of 756 registered DTx trials in China from 2014 to mid-2024, with 610 from ChiCTR and 146 from ClinicalTrials.gov (Fig. 1). Among these, 27 duplicate trials appearing in both registries were categorized under ChiCTR. Of these trials, 718 (95.0%) were interventional and 38 (5.0%) were observational (Table 1). 373 (49.3%) trials did not provide information on study phase. 316 (41.8%) trials were in early phase 1, representing the biggest proportion of all trials. The most common source of funding was government (277, 36.6%), followed by hospital/university (255, 33.7%), self-fund (176, 23.3%), and industry (42, 5.6%). Tertiary hospitals were involved in 594 (78.6%) trials, secondary hospitals in 42 (5.6%) trials, primary hospitals in 34 (4.5%) trials, community-based organizations in 72 (9.5%) trials, and others in 67 (8.7%) trials. 541 (71.6%) trials employed two arms. Only 77 (16.8%) studies used three or more.
Figure 2 shows a steady increase in the total number of DTx trials from 2014 to 2023. Notably, two significant surges were observed with a 200% growth rate in 2017 and a 46.7% growth rate in 2021 (Supplementary Table 2). The compound annual growth rate (CAGR) of the trials from 2018 to 2023 was 23.6%.
The provinces with the most DTx clinical trial sponsors (Fig. 3) were Beijing (n = 138), Shanghai (n = 107), Guangdong (n = 82), Sichuan (n = 77), and Zhejiang (n = 54). Two-thirds of the trial sponsors were located in eastern China.
This figure shows the number of registered DTx clinical trials in China’s mainland provinces, Hong Kong Special Administrative Region, and Macau Special Administrative Region, by the location of the trial sponsor, from 2014 to the first half of 2024. Registered trials in Taiwan were not specifically included in this search.
Characteristics of interventions in registered trials
Table 1 shows 339 (44.8%) trials involved a fully automated DTx, 296 (39.2%) involved a DTx-guided approach, and 121 (16.0%) involved DTx as adjunctive to physician-led services. Trials involving a DTx-guided approach outnumbered those with automatic DTx before 2019, but were overtaken by the latter from 2020 onwards (Supplementary Fig. 1). Most (397, 52.5%) trials focused on disease management, followed by 288 (38.1%) on treatment and 71 (9.4%) on prevention. However, trials on treatment saw a substantial increase, surpassing trials on prevention in 2018 and peaking at 44% in 2022 (Supplementary Table 3). The number of trials on prevention has remained low in the past decade (Supplementary Fig. 1). Most adjunctive models are designed for treatment purposes, while guided and fully automated models are predominantly designed for management purposes (Supplementary Fig. 2). Meanwhile, 658 (87.0%) studies are designed for non-hospital settings (i.e. community or home), with only 98 (13.0%) hospital-based studies (Table 1).
The five most commonly targeted medical condition categories of DTx trials were “mental, behavioral, or neurodevelopmental disorders” (n = 169, 22.4%), “circulatory system diseases” (n = 92, 12.2%), and “nervous system diseases” (n = 82, 10.8%), “endocrine, nutritional or metabolic diseases” (n = 68, 9.0%) and “neoplasms” (n = 64, 8.5%) (Supplementary Table 4). While treatment-oriented DTx trials predominantly focused on “mental, behavioral, or neurodevelopmental disorders”, and “disease of the nervous system”, the medical condition categories addressed by the management- and prevention-oriented DTx trials were more evenly distributed amongst the conditions (Supplementary Fig. 2). The three most studied disease areas up to 2019 were “circulatory system diseases”, “mental, behavioral, or neurodevelopmental disorders”, and “endocrine, nutritional, or metabolic diseases”, replaced by “mental, behavioral, or neurodevelopmental disorders”, “nervous system diseases”, and “neoplasms” afterwards (Supplementary Table 3).
China-based DTx trials encompassed a broad spectrum of disease stages, addressing prevention, treatment, and management across a variety of conditions and models of care. For example, trials assess the effectiveness of DTx in: reducing stress and preventing perinatal anxiety and depression through DTx-guided mindfulness exercises (ChiCTR2200061990), promoting healthy sleep habits with DTx-enabled, fully-automated training programs designed to prevent the progression of acute insomnia to chronic insomnia (ChiCTR2200066448), providing non-pharmacological pain relief for children in the PICU through provider-led DTx-supported virtual reality pain interventions (ChiCTR2300073767), improving social and communication skills in children with autism through DTx-enabled, fully-automated behavioral therapy (NCT0590155), as well as improving muscle strength through DTx-guided remote rehabilitation for patients with sarcopenia (ChiCTR2300077207).
Characteristics of participants
654 (86.5%) enrolled both male and female participants, as compared to 70 (9.3%) female-only trials, 28 (3.7%) male-only trials, and 4 trials leaving gender unspecified (Table 1). Age was not reported in 98 (13%) trials. 579 (76.6%) trials excluded children (>18 years) and 77 (10.2%) of these trials excluded the elderly (<60 years). Of the trials that did not exclude children or the elderly, 470 (62.2%) specified an upper age limit with a mean age of 75.3 (SD: 11.8) years (Supplementary Table 5). Only 9 (1.2%) trials specified the ethnicity of participants, all of which included only Han ethnicity.
Methodological issues of registered trials
The median sample size of the trials was 120 (IQR: 80–264) (Supplementary Table 6). However, the median sample size fell from 156 in 2018 to 113 in 2023. While the number of larger trials (with sample sizes over 200) has grown slowly, the much faster increase in smaller trials (with sample sizes below 200) has driven the overall decrease in median sample sizes (Supplementary Table 7). On average, the median duration was 18.4 months among trials that reported duration. 500 (66.1%) trials applied a study duration over 1 year, with 79 (10.5%) anticipated to finish within six months. The median duration decreased from 21.8 months in 2018 to 15.3 months in 2023. There were 614 (81.2%) single-center trials and 142 (18.8%) multi-center ones. The mean number of sites is 1.6, which dropped from 2.0 in 2018 to 1.5 in 2023. 89.0% of all trials reported randomization, with the proportion of randomized trials remaining stable over the years. Only 218 (28.8%) trials reported blinding, though the proportion improved significantly from 10.0% in 2018 to 44.9% in 2023. 724 (95.8%) trials did not use a digital placebo—usually an interface such as virtual reality and app, etc., similar to that of the studied DTx, but with different content.
Table 2 summarizes the risk of bias (RoB) of the 610 ChiCTR-registered studies (532 randomized and 78 non-randomized). Among these, 110 (18%) were at low overall RoB, 58 (9%) high overall RoB, and 442 (72%) unclear. 47% exhibited studies assessed are at unclear (43%) or high (4%) risk of selection bias. 55% of studies were at unclear (51%) or high (4%) RoB due to deviations from intended interventions. RoB in outcome measurement were unclear (37%) or high (2%) for 39% of the studies. RoB varied across the five most commonly studied medical condition categories, with “circulatory system diseases” and “endocrine, nutritional, or metabolic diseases” more likely than other groups to have low RoB.
Discussion
Previous studies about DTx trials neglected a key registry where most Chinese clinical trials are registered. We conducted a study of the DTx trial landscape in China to complement these findings about DTx trials globally. We observed a significant increase in the number of DTx trials over the past decade, consistent with the global trend, but at a faster growth rate than observed internationally19,20,22. However, over 70% of China-based trials received funding from government agencies, hospitals, and universities, suggesting that industrial investors are cautious about DTx.
Previous studies have given limited attention to intervention details such as models of care and specific intervention purposes. We identified a shift in the dominant focus of DTx trials from a primarily DTx-guided model of care to a fully automated model in China in recent years. While we were unable to isolate the effects of artificial intelligence (AI) applications in such a transition, there were clear signs of the important role of AI in recent trials. For example, DTx trials used AI to prompt patients about potentially inadequate preparation for colonoscopy and to guide further actions (ChiCTR2300071917), to improve effectiveness, quality, and safety of diabetic management in community (NCT06118671, ChiCTR2300068952), and to accelerate remote rehabilitation for patients recovering from isolated vertical meniscus tears (ChiCTR2300070582). Such a transition suggests a prospect that healthcare facilitated by DTx may be less dependent on professional healthcare providers in the future. Meanwhile, more than half of DTx trials overall involved a combined DTx-professional service (either DTx-guided or professional-led), emphasizing the importance of preparing healthcare professionals for working with DTx in a human-machine symbiosis.
Six out of every seven DTx trials are designed for community and home care settings, yet most were conducted by tertiary hospital researchers in eastern China. Such a hospital-centric model of DTx trial reflects the lack of primary care research capacity in China and the concentration of clinical research capacity in the eastern China. It potentially means neglect of the perspective of primary care and eventually restricts the ability of DTx to strengthen primary care, which would be the more suitable level of care to provide community-oriented, people-centered care. We also found a predominant focus on treatment and management, with consistently low proportions of trials on prevention, which contradicts the Healthy China policy’s emphasis on early disease prevention8.
Consistent with previous assessment of global DTx trials22, our study shows a high concentration of DTx trials related to mental, behavioral, or neurodevelopmental disorders. While circulation system diseases and cancers are the leading causes of disease burden in China23, they represent a relatively minor share within DTx trials. It appears that DTx may be more of a complementary role in healthcare delivery, rather than an alternative to current care model. As the care for the top causes of disease burden tends to be the ones better financed24,25,26, DTx and their trials may face restrictions in fundraising.
Previous research has shown some ethnic disparities in healthcare utilization and outcome, e.g. in maternal and child health, dental care, and diabetes and prediabetes27,28,29,30, indicating value in specific inclusion of ethnic minorities in clinical trials. Although DTx trials in China did not explicitly exclude ethnic minority groups, the lack of intentional inclusion may represent a missed opportunity to address their unique needs within these trials. The high prevalence of age-based exclusion criteria raises concerns related to ageism in clinical trials and casts doubts of applicability to a wider population.
The median sample size of Chinese DTx trials stayed above the global average, though it decreased over the past five years in contrast to the global rising trend22. The reduction in median sample size of the Chinese trials reflects the recent surge in the number of trials, many of which are at early stages with smaller sample sizes. The fast growth of number of trials with samples below 200 has contributed to a decline in median sample sizes. This trend may reflect the exploratory nature of recent DTx studies and/or smaller funding allocation per trial. Additionally, both trial durations and number of sites have decreased, with median durations in China being longer, and the median number of collaborators lower than global counterparts21. The proportion of randomized studies in China is higher, but the proportion of blinded studies is lower compared to global averages22. We observed a low (4%) use of digital placebos in DTx trials in China, mirroring the global situation19. This may be explained by the inherent challenges of implementing blinding and digital placebos effectively31. In general, the design quality of trials in China appears to be not inferior if not better than that of international ones, where only a small proportion of DTx studies globally is comprehensively well-designed19.
To the best of our knowledge, RoB assessment has not been conducted across DTx trials for all health conditions. Recognizing that reliance on published papers prevents timely assessment of the RoB of ongoing trials, we leveraged available registry data on ChiCTR for RoB assessment. Our findings suggest that most trials in China at unclear or high RoB in their design, consistent with the finding of a previous systematic review on digital mental health interventions by Lattie and colleagues, which analyzed 89 published studies and identified a moderate-to-severe risk of bias in many trials32.
Considering what we have found in the Chinese trials, there might be several implications for future practice and research. First, there is room for further improvement of trial designs to reduce biases, particularly in improving the use of blinding and digital placebo. Coordinated efforts to enhance DTx-specific research capacity are needed, such as formulating methodological guidelines for DTx studies and creating research training programs. Second, the capacity of primary care researchers should be strengthened to enable their leadership in DTx trials to reflect the perspective of community-oriented and primary care. Third, future trials in China should aim at more explicit inclusion of the western part of the country and ethnic minorities. Fourth, more DTx trials on preventive care and medical conditions that cause high disease burden should be encouraged. Fifth, as many DTx interventions still require professional support in service provision, health workers should be prepared to work in a human-machine symbiosis environment in the future.
Our studies have several limitations. First, we did not include data from trial registries other than the ChiCTR and Clinicaltrial.gov, which means a potential underestimation of China-based trials. Second, our assessment of RoB was not able to cover all China-based trials, due to the lack of relevant information on ClinicalTrials.gov regarding randomization and blinding procedure. Third, we were not able to compare the quality of trials registered on ChiCTR and ClinicalTrials.gov due to the limited scope of this study, while comparing trial characteristics across registries might offer valuable insights into inter-registry differences in trial quality. Last but not least, because we only considered the trial registration data but did not examine published protocols or papers, the assessment of the quality of study designs may not reflect the actual quality of trials as implemented.
Methods
Search strategy and selection criteria
We performed a search of DTx trials registered through the ClinicalTrials.gov (English) and Chinese Clinical Trials Registry (ChiCTR) (Chinese) from January 1, 2014, to June 30, 2024. We used the Chinese versions of the terms “digital therapeutics”, “telehealth”, “internet”, “mobile health”, “virtual reality”, “application”, and “mini program” to identify potential DTx trials on ChiCTR, and their English equivalents for the trials based in China on ClinicalTrials.gov (see detailed search terms in Supplementary Table 8).
After removing duplicate trials within and between each of the two registries, trials with active recruitment status or completed trials were included, i.e. those not “terminated”, “withdrawn”, “suspended”, “unknown”, or “no longer available”. After that, we assessed the full description of trials and excluded the ones, according to the DTx Value Assessment & Integration Guide (DTA, 2022)33, where: (1) the main purpose of intervention could not be categorized as disease prevention, treatment or management (i.e. general health promotion, wellness, fitness, or solely informing, monitoring, diagnosing, or offering insights to clinicians); (2) DTx was not delivered as part of the intervention to the patient (i.e. solely by a clinician or a medication independent of the software). Duplicated trials appearing in both registries were assigned to the ChiCTR category.
Variables, data extraction, and coding
Considering the information available on both registries, we assessed the priorities, quality, and inclusivity of trials using a set of indicators.
First, we evaluated a set of basic trial characteristics using the information provided on the registries: registration date, study type, study phase, source of funding, type of implementation site, number of arms, as well as the location of trial sponsors.
Second, we categorized the trials based on their textual description of intervention by: (1) model of care—“adjunctive” (i.e. provider-led DTx-supported), “guided” (i.e. DTx-led provider-supported) or “fully automated” (i.e. independent of provider involvement), according to a commonly used typology for behavioral intervention technology3; (2) purpose of intervention—“prevention”, “treatment” or “management”; and (3) application setting—“hospital” or “non-hospital”; (4) targeted medical condition category according to ICD-11 (see Supplementary Table 4).
Third, we assessed the quality of study design by reviewing the recorded sample size, duration of study, number of sites, randomization, blinding, and application of a digital placebo, as well as RoB. We assessed the RoB based on information available on the ChiCTR, namely, study design, randomization procedure, blinding procedure, inclusion criteria and exclusion criteria for participants, intervention and comparison, and primary outcomes (outcome, type of outcome, measurement time point of outcome, and measurement). Due to the lack of relevant information on ClinicalTrials.gov regarding randomization and blinding procedure, necessary for an RoB assessment, only studies registered on ChiCTR were included for our RoB assessment. In other words, we did not attempt to compare the RoB for trials registered in ChiCTR relative to those registered in ClinicalTrials.gov.
RoB was assessed using criteria from the Cochrane Handbook for Systematic Reviews of Interventions (version 6.4, 2023), specifically the RoB assessment for randomized trials and non-randomized studies34,35. The potential RoB was assessed in three key domains for both randomized and non-randomized studies: (1) selection bias (“bias arising from the randomization process” for randomized studies and “bias in the selection of participants into the study” for non-randomized studies); (2) bias due to deviations from intended interventions; (3) bias in the measurement of outcomes. RoB in each domain was rated as high, low, or unclear, based on trial descriptions. As our focus was trial design rather than result, we excluded the domains of biases “due to missing outcome data” and “in selection of the reported result” for all studies. For non-randomized studies, we further excluded biases “due to confounding” and “in classification of interventions”. Trials judged as low RoB in all domains were classified as low overall RoB; trials with any high risk were classified as high overall risk; and trials with any unclear risk were classified as unclear overall risk.
Fourth, we assessed the inclusivity of trials by examining the recorded gender, age, and ethnic composition of participants.
For variables other than RoB, two authors (X.X. and Z.W.) independently conducted data extraction and coding. The results of this initial extraction and coding were discussed between the two reviewers, with remaining disagreements resolved by discussion amongst the research team. Two authors (X.X. and W.L.) assessed RoB independently for each trial. Disagreements were resolved through discussion or by involving a third author (Z.W.).
Data acquisition, analysis, and visualization
We used Python 3.8.13 (Python Software Foundation, Delaware, USA) with the pandas package version 1.3.5 and seaborn package version 0.12.0 to download, code, and perform descriptive analyses of the data36,37. All codes are accessible in the following GitHub repository [https://github.com/kirihsia/An-assessment-of-digital-therapeutics-trials-in-China.git]. A Sankey diagram was used to visualize the changing characteristics of interventions and temporal trend of number of registered trials, model of care, purpose of intervention and disease category. Maps were drawn to describe the provincial distribution of trial sponsors within China.
Data availability
The datasets generately and analysed during the current study are available in the An-assessment-of-digital-therapeutics-trials-in-China repository, https://github.com/kirihsia/An-assessment-of-digital-therapeutics-trials-in-China.
Code availability
The underlying code for this study is available in An-assessment-of-digital-therapeutics-trials-in-China repository and can be accessed via this link https://github.com/kirihsia/An-assessment-of-digital-therapeutics-trials-in-China.
References
International Organization for Standardization. ISO/TR 11147:2023—Medical devices—Information to be provided by the manufacturer for medical devices with an intended purpose of supporting or providing a digital therapeutic. https://www.iso.org/obp/ui/#iso:std:iso:tr:11147:ed-1:v1:en (2023).
Digital Therapeutics Alliance. What is a DTx? https://dtxalliance.org/understanding-dtx/what-is-a-dtx/ (2024).
Hermes, E. D., Lyon, A. R., Schueller, S. M. & Glass, J. E. Measuring the Implementation of Behavioral Intervention Technologies: Recharacterization of Established Outcomes. J. Med Internet Res 21, e11752 (2019).
Digital Therapeutics Alliance. DTA policy positions. https://dtxalliance.org/advancing-dtx/dta-policy-positions/ (2024).
Vasdev, N., Gupta, T., Pawar, B., Bain, A. & Tekade, R. K. Navigating the future of health care with AI-driven digital therapeutics. Drug Discov. Today 29, 104110 (2024).
Sapanel, Y. et al. Economic evaluation associated with clinical-grade mobile app–based digital therapeutic interventions: systematic review. J. Med. Internet Res. 25, e47094 (2023).
Liu, T. et al. Cost-effectiveness analysis of digital therapeutics for home-based cardiac rehabilitation for patients with atrial fibrillation after catheter ablation. Digit Health 9, 20552076231211548 (2023).
Fujian Provincial People’s Government. Outline of the 14th Five-Year Plan (2021-2025) for National Economic and Social Development and Vision 2035. https://www.fujian.gov.cn/english/news/202108/t20210809_5665713.htm (2021).
Hainan Provincial Health Commission. Notice on organizing the application for Hainan Provincial Digital Therapeutics Clinical Trial Center. https://wst.hainan.gov.cn/swjw/ywdt/tzgg/202210/t20221014_3284669.html (2022).
People’s Daily Client. The first domestic group standard for digital therapeutics was established in Tianjin Economic-Technological Development Area. Tianjin Economic-Technological Development Area. https://www.teda.gov.cn/contents/19/32382.html (2024).
Zhejiang Medical Products Administration. Zhejiang Medical Device Review Center provides remote precision services to digital therapeutics industrial park enterprises. Zhejiang Medical Products Administration. https://mpa.zj.gov.cn/art/2022/4/22/art_1228989351_58928659.html (2022).
Phan, P., Mitragotri, S. & Zhao, Z. Digital therapeutics in the clinic. Bioeng. Transl. Med. 8, e10536 (2023).
Digital Therapeutics Alliance. U.S. regulatory and reimbursement pathways. https://dtxalliance.org/wp-content/uploads/2022/01/US-Regulatory-and-Reimbursement-Pathways.pdf (2022).
Meng, Q., Fang, H., Liu, X., Yuan, B. & Xu, J. Consolidating the social health insurance schemes in China: towards an equitable and efficient health system. Lancet 386, 1484–1492 (2015).
Chinese Society of Digital Psychosomatic Medicine. Consensus Panel on Digital Therapeutics for Insomnia. Zhonghua Yi Xue Za Zhi (Chin.) 104, 650–661 (2024).
China Sleep Research Society. Expert Consensus on digital therapies for insomnia in China. Chin. Gen. Pract. (Chin.) 27, 381–390 (2024).
Chinese Association of Drug Abuse Prevention and Treatment. Consensus Panel on Digital Therapeutics for Addictive Disorders. Expert consensus on digital therapeutics for addictive disorders. Natl Med. J. China (Chin.) 103, 2757–2764 (2023).
Chinese Society of Dementia and Cognitive Impairment. Consensus Panel on Digital Therapeutics for Cognitive Impairment. [Chinese expert consensus on digital therapeutics for cognitive impairment (2023 edition)]. Zhonghua Yi Xue Za Zhi (Chin.) 103, 640–647 (2023).
Kumar, A. et al. Studies of prescription digital therapeutics often lack rigor and inclusivity. Health Aff. (Millwood) 42, 1559–1567 (2023).
Yao, H. et al. A comprehensive survey of the clinical trial Landscape on digital therapeutics. Heliyon 10, e36115 (2024).
Miao, B. Y. et al. Characterisation of digital therapeutic clinical trials: a systematic review with natural language processing. Lancet Digit. Health 6, e222–e229 (2024).
Masanneck, L. & Stern, A. D. Tracing digital therapeutics research across medical specialties: evidence from ClinicalTrials.gov. Clin. Pharm. Ther. 116, 177–185 (2024).
World Health Organization. Global Health Estimates 2021: Disease burden by Cause, Age, Sex, by Country and by Region, 2000–2021. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/global-health-estimates-leading-causes-of-dalys (2024).
Ma, J. et al. Estimating the economic burden of stroke in China: a cost-of-illness study. BMJ Open 14, e080634 (2024).
Lu, M. et al. Health care costs of cardiovascular disease in China: a machine learning-based cross-sectional study. Front. Public Health 11, 1301276 (2023).
Huang, H. Y. et al. Expenditure and financial burden for the diagnosis and treatment of colorectal cancer in China: a hospital-based, multicenter, cross-sectional survey. Chin. J. Cancer 36, 41 (2017).
Huang, Y. et al. Ethnicity and maternal and child health outcomes and service coverage in western China: a systematic review and meta-analysis. Lancet Glob. Health 6, e39–e56 (2018).
Wu, Y. et al. Ethnic disparities in stillbirth risk in Yunnan, China: a prospective cohort study, 2010–2018. BMC Public Health 21, 136 (2021).
Wu, S. C. et al. Ethnic disparities in dental caries among adolescents in China. J. Dent. Res. 100, 496–506 (2021).
Wang, L. et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. J. Am. Med. Assoc. 317, 2515–2523 (2017).
Molnar, A. Prescription Digital Therapeutics: Assertions. Health Aff. (Millwood) 43, 897 (2024).
Lattie, E. G. et al. Digital mental health interventions for depression, anxiety, and enhancement of psychological well-being among college students: systematic review. J. Med. Internet Res. 21, e12869 (2019).
Digital Therapeutics Alliance. DTx value assessment & integration guide. https://dtxalliance.org/wp-content/uploads/2022/05/DTx-Value-Assessment-Guide_May-2022.pdf (2022).
Higgins, J. P. T. et al. Chapter 8: Assessing risk of bias in a randomized trial. In Higgins, J.P.T. et al. Cochrane Handbook for Systematic Reviews of Interventions (Version 6.5). https://training.cochrane.org/handbook (2019).
Sterne, J. A. C., Hernán, M. A., McAleenan, A., Reeves, B. C. & Higgins, J. P. T. Chapter 25: Assessing risk of bias in a non-randomized study. In Higgins, J.P.T. et al. Cochrane Handbook for Systematic Reviews of Interventions (Version 6.5). https://training.cochrane.org/handbook (2019).
Pandas Development Team. Pandas (Version 1.3.3) https://doi.org/10.5281/zenodo.3509134 (2021).
Waskom, M. L. seaborn: Statistical data visualization. J. Open Source Softw. 6, 3021 (2021).
Acknowledgements
We would like to express our sincere gratitude to Edward J. Mullen, Ph.D., Willma and Albert Musher Professor Emeritus, Columbia University in the City of New York, for his thorough review and invaluable advice throughout the revision process. This study received no funding.
Author information
Authors and Affiliations
Contributions
Conceptualization: Z.W., J.X., X.X., W.L.; Literature review: Z.W., Y.Y., J.X.; Methodology: Z.W., X.X., W.L., J.X.; Data collection: X.X., Z.W., Y.Y.; Data analysis: X.X., Z.W., J.X., W.L.; Interpretation: J.X., Z.W.; Visualization: X.X., Z.W.; Drafting of paper: Z.W., X.X., W.L., J.X.; Supervision: J.X.; Revision of paper: J.X., Z.W., X.X., W.L.; Directly accessed and verified the underlying data reported in the paper: X.X., Z.W., W.L.; Full access to all the data in the study and accept responsibility to submit for publication: All authors. Review and approval of the final paper: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Z., Xia, X., Lu, W. et al. Assessment of priorities, quality, and inclusivity of digital therapeutics trials in China. npj Digit. Med. 8, 83 (2025). https://doi.org/10.1038/s41746-025-01477-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-025-01477-6