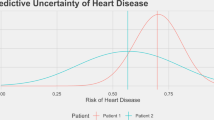
Abstract
Applications of machine learning are becoming increasingly common in medicine and healthcare, enabling more accurate predictive models. However, this often comes at the cost of interpretability, limiting the clinical impact of machine learning methods. To realize the potential of machine learning in healthcare, it is critical to understand such models from the perspective of multiple stakeholders and various angles, necessitating different types of explanation. In this Perspective, we explore five fundamentally different types of post-hoc machine learning interpretability. We highlight the different types of information that they provide, and describe when each can be useful. We examine the various stakeholders in healthcare, delving into their specific objectives, requirements and goals. We discuss how current notions of interpretability can help meet these and what is required for each stakeholder to make machine learning models clinically impactful. Finally, to facilitate adoption, we release an open-source interpretability library containing implementations of the different types of interpretability, including tools for visualizing and exploring the explanations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Code availability
The open-source software package is available via Github at https://github.com/vanderschaarlab/Interpretability.
References
Topol, E. J. High-performance medicine: the convergence of human and artificial intelligence. Nat. Med. 25, 44–56 (2019).
Volovici, V., Syn, N. L., Ercole, A., Zhao, J. J. & Liu, N. Steps to avoid overuse and misuse of machine learning in clinical research. Nat. Med. 28, 1996–1999 (2022).
Caruana, R. et al. Intelligible models for healthcare: predicting pneumonia risk and hospital 30-day readmission. In Proc. 21th ACM SIGKDD International Conference on Knowledge Discovery and Data Mining 1721–1730 (ACM, 2015).
Winkler, J. K. et al. Association between surgical skin markings in dermoscopic images and diagnostic performance of a deep learning convolutional neural network for melanoma recognition. JAMA Dermatol. 155, 1135–1141 (2019).
Amann, J. et al. Explainability for artificial intelligence in healthcare: a multidisciplinary perspective. BMC Med. Inf. Decis. Making 20, 310 (2020).
Rajpurkar, P., Chen, E., Banerjee, O. & Topol, E. J. AI in health and medicine. Nat. Med. 28, 31–38 (2022).
Yoon, C. H., Torrance, R. & Scheinerman, N. Machine learning in medicine: should the pursuit of enhanced interpretability be abandoned? J. Med. Ethics 48, 581–585 (2022).
Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD) (Food and Drug Administration, 2019).
Mourby, M., Ó Cathaoir, K. & Collin, C. B. Transparency of machine-learning in healthcare: the GDPR & European health law. Comput. Law Secur. Rev. 43, 105611 (2021).
Rudin, C. Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nat. Mach. Intell. 1, 206–215 (2019).
Esteva, A. et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 542, 115–118 (2017).
Gulshan, V. et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 316, 2402–2410 (2016).
Brown, T. B. et al. Language models are few-shot learners. Adv. Neur. Inf. Process. Syst. 33, 1877–1901 (2020).
Jiang, L. Y. et al. Health system-scale language models are all-purpose prediction engines. Nature (2023).
Soenksen, L. R. et al. Integrated multimodal artificial intelligence framework for healthcare applications. npj Digit. Med. 5, 149 (2022).
Rajkomar, A. et al. Scalable and accurate deep learning with electronic health records. npj Digit. Med. 1, 18 (2018).
Alaa, A. M., Bolton, T., Di Angelantonio, E., Rudd, J. H. F. & van der Schaar, M. Cardiovascular disease risk prediction using automated machine learning: a prospective study of 423,604 UK Biobank participants. PLoS ONE 14, e0213653 (2019).
Lee, C., Light, A., Saveliev, E. S., van der Schaar, M. & Gnanapragasam, V. J. Developing machine learning algorithms for dynamic estimation of progression during active surveillance for prostate cancer. npj Digit. Med. 5, 110 (2022).
Akbilgic, O. & Davis, R. L. The promise of machine learning: when will it be delivered? J. Card. Fail. 25, 484–485 (2019).
Schulz, M.-A. et al. Different scaling of linear models and deep learning in UKBiobank brain images versus machine-learning datasets. Nat. Commun. 11, 4238 (2020).
London, A. J. Artificial intelligence and black-box medical decisions: accuracy versus explainability. Hastings Cent. Rep. 49, 15–21 (2019).
Biran, O. & Cotton, C. Explanation and justification in machine learning: a survey. IJCAI-17 Workshop on Explainable AI (XAI) 8, 8–13 (2017).
Miller, T. Explanation in artificial intelligence: insights from the social sciences. Artif. Intell. 267, 1–38 (2019).
Lipton, Z. C. The mythos of model interpretability: In machine learning, the concept of interpretability is both important and slippery. Queue 16, 31–57 (2018).
Ribeiro, M. T., Singh, S. & Guestrin, C. ‘Why should I trust you?’ Explaining the predictions of any classifier. In Proc. 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining 1135–1144 (ACM, 2016).
Lundberg, S. M. & Lee, S.-I. A unified approach to interpreting model predictions. Adv. Neur. Inf. Process. Syst. 30, 4765–4774 (2017).
Sundararajan, M., Taly, A. & Yan, Q. Axiomatic attribution for deep networks. In Proc. 34th International Conference on Machine Learning 3319–3328 (PMLR, 2017).
Friedman, J. H. Greedy function approximation: a gradient boosting machine. Ann. Stat. 29, 1189–1232 (2001).
Breiman, L. Random forests. Mach. Learn. 45, 5–32 (2001).
Fisher, A., Rudin, C. & Dominici, F. All models are wrong, but many are useful: learning a variable’s importance by studying an entire class of prediction models simultaneously. J. Mach. Learn. Res. 20, 1–81 (2019).
Imrie, F., Norcliffe, A. L. I., Lio, P. & van der Schaar, M. Composite feature selection using deep ensembles. Adv. Neur. Inf. Process. Syst. 35, 36142–36160 (2022).
Aamodt, A. & Plaza, E. Case-based reasoning: foundational issues, methodological variations, and system approaches. AI Commun. 7, 39–59 (1994).
Crabbe, J., Qian, Z., Imrie, F. & van der Schaar, M. Explaining latent representations with a corpus of examples. Adv. Neur. Inf. Process. Syst. 34, 12154–12166 (2021).
Jeyakumar, J. V., Noor, J., Cheng, Y.-H., Garcia, L. & Srivastava, M. How can I explain this to you? An empirical study of deep neural network explanation methods. Adv. Neur. Inf. Process. Syst. 33, 4211–4222 (2020).
Wiesenfeld, B. M., Aphinyanaphongs, Y. & Nov, O. AI model transferability in healthcare: a sociotechnical perspective. Nat. Mach. Intell. 4, 807–809 (2022).
Kim, B. et al. Interpretability beyond feature attribution: quantitative testing with concept activation vectors (TCAV). In Proc. 35th International Conference on Machine Learning 2668–2677 (PMLR, 2018).
Crabbé, J. & van der Schaar, M. Concept activation regions: A generalized framework for concept-based explanations. Adv. Neur. Inf. Process. Syst. 35, 2590–2607 (2022).
Ghorbani, A., Wexler, J., Zou, J. Y. & Kim, B. Towards automatic concept-based explanations. Adv. Neur. Inf. Process. Syst. 32, 9277–9286 (2019).
Thabtah, F. A review of associative classification mining. Knowl. Eng. Rev. 22, 37–65 (2007).
Luo, G. Automatically explaining machine learning prediction results: a demonstration on type 2 diabetes risk prediction. Health Inf. Sci. Syst. 4, 2 (2016).
Alaa, A. M. & van der Schaar, M. Prognostication and risk factors for cystic fibrosis via automated machine learning. Sci. Rep. 8, 11242 (2018).
Alaa, A. M. & van der Schaar, M. Demystifying black-box models with symbolic metamodels. Adv. Neur. Inf. Process. Syst. 32, 11304–11314 (2019).
Crabbe, J., Zhang, Y., Zame, W. R. & van der Schaar, M. Learning outside the black-box: the pursuit of interpretable models. Adv. Neur. Inf. Process. Syst. 33, 17838–17849 (2020).
Min, F., Hu, Q. & Zhu, W. Feature selection with test cost constraint. Int. J. Approx. Reason. 55, 167–179 (2014).
Geirhos, R. et al. Shortcut learning in deep neural networks. Nat. Mach. Intell. 2, 665–673 (2020).
DeGrave, A. J., Janizek, J. D. & Lee, S.-I. AI for radiographic COVID-19 detection selects shortcuts over signal. Nat. Mach. Intell. 3, 610–619 (2021).
Roberts, M. et al. Common pitfalls and recommendations for using machine learning to detect and prognosticate for COVID-19 using chest radiographs and CT scans. Nat. Mach. Intell. 3, 199–217 (2021).
Ko, J. et al. Machine learning to detect signatures of disease in liquid biopsies—a user’s guide. Lab Chip 18, 395–405 (2018).
Wang, D. et al. Identification of differentially expressed genes between original breast cancer and xenograft using machine learning algorithms. Genes 9, 155 (2018).
Wu, E. et al. How medical AI devices are evaluated: limitations and recommendations from an analysis of FDA approvals. Nat. Med. 27, 582–584 (2021).
Regulation 2016/679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons with Regard to the Processing of Personal Data and on the Free Movement of Such Data, and Repealing Directive 95/46/EC (General Data Protection Regulation) (EUR, 2016).
Rajkomar, A., Hardt, M., Howell, M. D., Corrado, G. & Chin, M. H. Ensuring fairness in machine learning to advance health equity. Ann. Int. Med. 169, 866–872 (2018).
Tomašev, N. et al. AI for social good: unlocking the opportunity for positive impact. Nat. Commun. 11, 2468 (2020).
Kattan, M. W. et al. American Joint Committee on Cancer acceptance criteria for inclusion of risk models for individualized prognosis in the practice of precision medicine. CA Cancer J. Clin. 66, 370–374 (2016).
Alaa, A. M., Gurdasani, D., Harris, A. L., Rashbass, J. & van der Schaar, M. Machine learning to guide the use of adjuvant therapies for breast cancer. Nat. Mach. Intell. 3, 716–726 (2021).
Van der Velden, B. H., Kuijf, H. J., Gilhuijs, K. G. & Viergever, M. A. Explainable artificial intelligence (XAI) in deep learning-based medical image analysis. Med. Image Anal. 79, 102470 (2022).
Rajpurkar, P. et al. CheXaid: deep learning assistance for physician diagnosis of tuberculosis using chest x-rays in patients with HIV. npj Digit. Med. 3, 115 (2020).
Rudin, C. Why black box machine learning should be avoided for high-stakes decisions, in brief. Nat. Rev. Methods Primers 2, 81 (2022).
Rudin, C., Wang, C. & Coker, B. The age of secrecy and unfairness in recidivism prediction. Harvard Data Sci. Rev. 2, https://hdsr.mitpress.mit.edu/pub/7z10o269 (2020).
Ghassemi, M., Oakden-Rayner, L. & Beam, A. L. The false hope of current approaches to explainable artificial intelligence in health care. Lancet Digit. Health 3, e745–e750 (2021).
Reyes, M. et al. On the interpretability of artificial intelligence in radiology: challenges and opportunities. Radiol. Artif. Intell. 2, e190043 (2020).
Reddy, S. Explainability and artificial intelligence in medicine. Lancet Digit. Health 4, e214–e215 (2022).
Arcadu, F. et al. Deep learning algorithm predicts diabetic retinopathy progression in individual patients. npj Digit. Med. 2, 92 (2019).
Pierson, E., Cutler, D. M., Leskovec, J., Mullainathan, S. & Obermeyer, Z. An algorithmic approach to reducing unexplained pain disparities in underserved populations. Nat. Med. 27, 136–140 (2021).
van der Schaar, M. & Maxfield, N. Making machine learning interpretable: a dialog with clinicians. Van der Schaar Lab https://www.vanderschaar-lab.com/making-machine-learning-interpretable-a-dialog-with-clinicians/ (2021).
Dandl, S., Molnar, C., Binder, M. & Bischl, B. Multi-objective counterfactual explanations. In International Conference on Parallel Problem Solving from Nature 448–469 (Springer, 2020).
Acknowledgements
F.I. and M.v.d.S. are supported by the National Science Foundation (NSF) under grant number 1722516. M.vdS. is also supported by the Office of Naval Research (ONR).
Author information
Authors and Affiliations
Contributions
F.I. and M.v.d.S. conceptualized the manuscript. R.D. developed the interpretability package. F.I. wrote the original draft of the manuscript, and all authors contributed to editing and revising it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Machine Intelligence thanks Victor Volovici, Po-Hsuan Cameron Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Imrie, F., Davis, R. & van der Schaar, M. Multiple stakeholders drive diverse interpretability requirements for machine learning in healthcare. Nat Mach Intell 5, 824–829 (2023). https://doi.org/10.1038/s42256-023-00698-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42256-023-00698-2