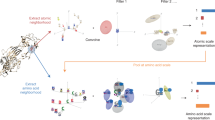
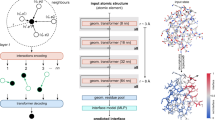
Abstract
Computational protein-binding studies are widely used to investigate fundamental biological processes and facilitate the development of modern drugs, vaccines and therapeutics. Scoring functions aim to assess and rank the binding strength of the predicted protein complex. However, accurate scoring of protein binding interfaces remains a challenge. Here we show that our evaluating Protein binding Interfaces with Transformer Networks (PIsToN) approach can distinguish native-like protein complexes from incorrect conformations. Protein interfaces are transformed into a collection of two-dimensional images (interface maps), each corresponding to a geometric or biochemical property. Pixel intensities represent the feature values. A neural network was adapted from a popular vision transformer with several enhancements: a hybrid component to accept empirical-based energy terms, a multi-attention module to highlight essential features and binding sites, and the use of contrastive learning for better ranking performance. The resulting PIsToN model substantially outperforms state-of-the-art scoring functions on well-known datasets.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The lists of training/testing protein complexes and pre-computed interface maps are available on Zenodo at https://doi.org/10.5281/zenodo.7948337.
Code availability
The PIsToN software and benchmark datasets are available under license from https://biorg.cis.fiu.edu/piston/. The specific version of PIsToN used in this study is readily available for access via Zenodo84.
References
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Callaway, E. After AlphaFold: protein-folding contest seeks next big breakthrough. Nature 604, 234–238 (2022).
Vakser, I. A. Protein–protein docking: from interaction to interactome. Biophys. J. 107, 1785–1793 (2014).
Shin, W.-H., Christoffer, C. W. & Kihara, D. In silico structure-based approaches to discover protein–protein interaction-targeting drugs. Methods 131, 22–32 (2017).
Meng, X.-Y., Zhang, H.-X., Mezei, M. & Cui, M. Molecular docking: a powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug Des. 7, 146–157 (2011).
Scior, T. et al. Recognizing pitfalls in virtual screening: a critical review. J. Chem. Inf. Model. 52, 867–881 (2012).
Gupta, M., Sharma, R. & Kumar, A. Docking techniques in pharmacology: how much promising? Comput. Biol. Chem. 76, 210–217 (2018).
Huang, S.-Y. Search strategies and evaluation in protein–protein docking: principles, advances and challenges. Drug Discov. Today 19, 1081–1096 (2014).
Shen, C. et al. From machine learning to deep learning: advances in scoring functions for protein–ligand docking. Wiley Interdiscip. Rev. Comput. Mol. Sci. 10, e1429 (2020).
Moal, I. H., Torchala, M., Bates, P. A. & Fernández-Recio, J. The scoring of poses in protein–protein docking: current capabilities and future directions. BMC Bioinformatics 14, 286 (2013).
Adeshina, Y. O., Deeds, E. J. & Karanicolas, J. Machine learning classification can reduce false positives in structure-based virtual screening. Proc. Natl Acad. Sci. USA 117, 18477–18488 (2020).
Li, J., Fu, A. & Zhang, L. An overview of scoring functions used for protein–ligand interactions in molecular docking. Interdiscip. Sci. Comput. Life Sci. 11, 320–328 (2019).
Kortemme, T. & Baker, D. A simple physical model for binding energy hot spots in protein–protein complexes. Proc. Natl Acad. Sci. USA 99, 14116–14121 (2002).
Liu, X., Peng, L. & Zhang, J. Z. Accurate and efficient calculation of protein–protein binding free energy-interaction entropy with residue type-specific dielectric constants. J. Chem. Inf. Model. 59, 272–281 (2018).
Huang, S.-Y., Grinter, S. Z. & Zou, X. Scoring functions and their evaluation methods for protein–ligand docking: recent advances and future directions. Phys. Chem. Chem. Phys. 12, 12899–12908 (2010).
Durham, E., Dorr, B., Woetzel, N., Staritzbichler, R. & Meiler, J. Solvent accessible surface area approximations for rapid and accurate protein structure prediction. J. Mol. Model. 15, 1093–1108 (2009).
Eldridge, M. D., Murray, C. W., Auton, T. R., Paolini, G. V. & Mee, R. P. Empirical scoring functions: I. The development of a fast empirical scoring function to estimate the binding affinity of ligands in receptor complexes. J. Comput. Aided Mol. Des. 11, 425–445 (1997).
Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010).
Chen, Y.-C. Beware of docking! Trends Pharmacol. Sci. 36, 78–95 (2015).
Muley, L. et al. Enhancement of hydrophobic interactions and hydrogen bond strength by cooperativity: synthesis, modeling, and molecular dynamics simulations of a congeneric series of thrombin inhibitors. J. Med. Chem. 53, 2126–2135 (2010).
Liu, J. & Wang, R. Classification of current scoring functions. J. Chem. Inf. Mode. 55, 475–482 (2015).
Kinnings, S. L. et al. A machine learning-based method to improve docking scoring functions and its application to drug repurposing. J. Chem. Inf. Model. 51, 408–419 (2011).
Das, S. & Chakrabarti, S. Classification and prediction of protein–protein interaction interface using machine learning algorithm. Sci. Rep. 11, 1761 (2021).
Zilian, D. & Sotriffer, C. A. SFCscore(RF): a random forest-based scoring function for improved affinity prediction of protein–ligand complexes. J. Chem. Inf. Model. 53, 1923–1933 (2013).
Durrant, J. D. & McCammon, J. A. NNScore: a neural-network-based scoring function for the characterization of protein–ligand complexes. J. Chem. Inf. Model. 50, 1865–1871 (2010).
Ballester, P. J. & Mitchell, J. B. A machine learning approach to predicting protein–ligand binding affinity with applications to molecular docking. Bioinformatics 26, 1169–1175 (2010).
Li, H., Leung, K.-S., Wong, M.-H. & Ballester, P. J. Substituting random forest for multiple linear regression improves binding affinity prediction of scoring functions: Cyscore as a case study. BMC Bioinformatics 15, 291 (2014).
Li, Y., Zhang, X. & Cao, D. The role of shape complementarity in the protein–protein interactions. Sci. Rep. 3, 3271 (2013).
Wallach, I., Dzamba, M. & Heifets, A. AtomNet: a deep convolutional neural network for bioactivity prediction in structure-based drug discovery. Preprint at https://arxiv.org/abs/1510.02855 (2015).
Imrie, F., Bradley, A. R., van der Schaar, M. & Deane, C. M. Protein family-specific models using deep neural networks and transfer learning improve virtual screening and highlight the need for more data. J. Chem. Inf. Model. 58, 2319–2330 (2018).
Balci, A. et al. DeepInterface: protein–protein interface validation using 3D convolutional neural networks. Preprint at bioRxiv https://doi.org/10.1101/617506 (2019).
Wang, X., Terashi, G., Christoffer, C. W., Zhu, M. & Kihara, D. Protein docking model evaluation by 3D deep convolutional neural networks. Bioinformatics 36, 2113–2118 (2020).
Renaud, N. et al. DeepRank: a deep learning framework for data mining 3D protein-protein interfaces. Nat. Commun. 12, 7068 (2021).
Mohseni Behbahani, Y., Crouzet, S., Laine, E. & Carbone, A. Deep local analysis evaluates protein docking conformations with locally oriented cubes. Bioinformatics 38, 4505–4512 (2022).
Kumawat, S. & Raman, S. LP-3DCNN: unveiling local phase in 3D convolutional neural networks. In Proc. IEEE/CVF Conference on Computer Vision and Pattern Recognition 4903–4912 (IEEE, 2019).
Geng, C. et al. iScore: a novel graph kernel-based function for scoring protein–protein docking models. Bioinformatics 36, 112–121 (2020).
Budowski-Tal, I., Kolodny, R. & Mandel-Gutfreund, Y. A novel geometry-based approach to infer protein interface similarity. Sci. Rep. 8, 1–10 (2018).
Wang, X., Flannery, S. T. & Kihara, D. Protein docking model evaluation by graph neural networks. Front. Mol. Biosci. 8, 647915 (2021).
Réau, M., Renaud, N., Xue, L. C. & Bonvin, A. M. DeepRank-GNN: a graph neural network framework to learn patterns in protein–protein interfaces. Bioinformatics 39, 759 (2023).
Gainza, P. et al. Deciphering interaction fingerprints from protein molecular surfaces using geometric deep learning. Nat. Methods 17, 184–192 (2020).
Sverrisson, F., Feydy, J., Correia, B. E. & Bronstein, M. M. Fast end-to-end learning on protein surfaces. In Proc. IEEE/CVF Conference on Computer Vision and Pattern Recognition 15272–15281 (IEEE, 2021).
Zhai, X., Kolesnikov, A., Houlsby, N. & Beyer, L. Scaling vision transformers. In Proc. IEEE/CVF Conference on Computer Vision and Pattern Recognition 12104–12113 (IEEE, 2022).
Dosovitskiy, A. et al. An image is worth 16x16 words: transformers for image recognition at scale. 9th International Conference on Learning Representations, ICLR (2020).
Yan, Y., Tao, H., He, J. & Huang, S.-Y. The HDOCK server for integrated protein–protein docking. Nat. Protoc. 15, 1829–1852 (2020).
Janin, J. et al. CAPRI: a critical assessment of predicted interactions. Proteins 52, 2–9 (2003).
Khosla, P. et al. Supervised contrastive learning. Ad. Neural Inf. Process. Syst. 33, 18661–18673 (2020).
Chen, C. et al. This looks like that: deep learning for interpretable image recognition. Adv. Neural Inf. Process. Systems 32, 8930–8941 (2019).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Jones, D. T. Setting the standards for machine learning in biology. Nat. Rev. Mol. Cell Biol. 20, 659–660 (2019).
Jeong, J.-J. et al. Characterization of the cupin-type phosphoglucose isomerase from the hyperthermophilic archaeon Thermococcus litoralis. FEBS Lett. 535, 200–204 (2003).
Dominguez, C., Boelens, R. & Bonvin, A. M. HADDOCK: a protein–protein docking approach based on biochemical or biophysical information. J. Am. Chem. Soc. 125, 1731–1737 (2003).
Wang, R., Fang, X., Lu, Y. & Wang, S. The PDBbind database: collection of binding affinities for protein–ligand complexes with known three-dimensional structures. J. Med. Chem. 47, 2977–2980 (2004).
Evans, R. et al. Protein complex prediction with AlphaFold-Multimer. Preprint at bioRxiv https://doi.org/10.1101/2021.10.04.463034 (2021).
Stebliankin, V. et al. EMoMiS: a pipeline for epitope-based molecular mimicry search in protein structures with applications to SARS-CoV-2. Preprint at bioRxiv https://doi.org/10.1101/2022.02.05.479274 (2022).
Balbin, C. A. et al. Epitopedia: identifying molecular mimicry between pathogens and known immune epitopes. ImmunoInformatics 9, 100023 (2023).
Berman, H. M. et al. The Protein Data Bank. Nucleic Acids Res. 28, 235–242 (2000).
Valdes, C. et al. Microbiome maps: Hilbert curve visualizations of metagenomic profiles. Front. Bioinformatics 3, 1154588 (2023).
Andrusier, N., Nussinov, R. & Wolfson, H. J. FireDock: fast interaction refinement in molecular docking. Proteins 69, 139–159 (2007).
Gray, J. J. et al. Protein–protein docking with simultaneous optimization of rigid-body displacement and side-chain conformations. J. Mol. Biol. 331, 281–299 (2003).
Zhang, C., Vasmatzis, G., Cornette, J. L. & DeLisi, C. Determination of atomic desolvation energies from the structures of crystallized proteins. J. Mol. Biol. 267, 707–726 (1997).
Dunbrack Jr, R. L. & Cohen, F. E. Bayesian statistical analysis of protein side-chain rotamer preferences. Protein Sci. 6, 1661–1681 (1997).
Neria, E., Fischer, S. & Karplus, M. Simulation of activation free energies in molecular systems. J. Chem. Phys. 105, 1902–1921 (1996).
Crowley, P. B. & Golovin, A. Cation–π interactions in protein–protein interfaces. Proteins59, 231–239 (2005).
Kabsch, W. & Sander, C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22, 2577–2637 (1983).
Touw, W. G. et al. A series of PDB-related databanks for everyday needs. Nucleic Acids Res. 43, 364–368 (2015).
Mead, A. Review of the development of multidimensional scaling methods. J. R. Stat. Soc. Ser. D 41, 27–39 (1992).
Ng, A. Y. Feature selection, L1 vs. L2 regularization, and rotational invariance. In Proc. Twenty-first International Conference on Machine Learning 78 (Association for Computing Machinery, 2004).
Loshchilov, I. & Hutter, F. Decoupled weight decay regularization. 7th International Conference on Learning Representations, ICLR, 6–9 (2017).
Lensink, M. F. & Wodak, S. J. Score_set: a CAPRI benchmark for scoring protein complexes. Proteins 82, 3163–3169 (2014).
Zhang, C., Shine, M., Pyle, A. M. & Zhang, Y. US-align: universal structure alignments of proteins, nucleic acids, and macromolecular complexes. Nat. Methods 19, 1109–1115 (2022).
Cheng, T. M.-K., Blundell, T. L. & Fernandez-Recio, J. pyDock: electrostatics and desolvation for effective scoring of rigid-body protein–protein docking. Proteins 68, 503–515 (2007).
Leman, J. K. et al. Macromolecular modeling and design in Rosetta: recent methods and frameworks. Nat. Methods 17, 665–680 (2020).
Pierce, B. & Weng, Z. A combination of rescoring and refinement significantly improves protein docking performance. Proteins 72, 270–279 (2008).
Viswanath, S., Ravikant, D. & Elber, R. Improving ranking of models for protein complexes with side chain modeling and atomic potentials. Proteins 81, 592–606 (2013).
Pons, C., Talavera, D., De La Cruz, X., Orozco, M. & Fernandez-Recio, J. Scoring by intermolecular pairwise propensities of exposed residues (SIPPER): a new efficient potential for protein–protein docking. J. Chem. Inf. Model. 51, 370–377 (2011).
Ravikant, D. & Elber, R. Pie-efficient filters and coarse grained potentials for unbound protein–protein docking. Proteins 78, 400–419 (2010).
Moal, I. H., Jiménez-García, B. & Fernández-Recio, J. CCharPPI web server: computational characterization of protein–protein interactions from structure. Bioinformatics 31, 123–125 (2015).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020).
Renaud, N. & Geng, C. The pdb2sql Python package: parsing, manipulation and analysis of PDB files using SQL queries. J. Open Source Softw. 5, 2077 (2020).
Paszke, A. et al. PyTorch: an imperative style, high-performance deep learning library. Adv. Neural Inf. Processing Syst. 32, 8024–8035 (2019).
Collaborative Data Science (Plotly Technologies, 2015); https://plot.ly
Camacho, C. et al. BLAST+: architecture and applications. BMC Bioinformatics 10, 421 (2009).
The PyMOL Molecular Graphics System, Version 1.8 (Schrödinger, LLC, 2015).
Stebliankin, V. stebliankin/piston: PIsToN (v.1.0.0). Zenodo https://doi.org/10.5281/zenodo.8102876 (2023).
Shore, D., Issafras, H., Landais, E., Teyton, L. & Wilson, I. The crystal structure of CD8 in complex with YTS156. 7.7 Fab and interaction with other CD8 antibodies define the binding mode of CD8 αβ to MHC class I. J. Mol. Biol. 384, 1190–1202 (2008).
Acknowledgements
We thank the members of the Bioinformatics Research Group (BioRG) at FIU for their valuable feedback and comments. This work grew out of a project that was supported by grants from the National Science Foundation (CNS-2037374 and OAC-2118329). V.S. gratefully acknowledges funding for graduate assistantships from the Department of Education, the National Science Foundation, and the Knight Foundation School of Computing and Information Sciences
Author information
Authors and Affiliations
Contributions
G.N. conceptualized and supervised the project. V.S. designed, implemented and tested the PIsToN framework. V.S. and A.S. executed benchmarks. P.B., J.S., P.C. and K.M. assisted in the feature extraction design and interpretations. V.S., A.S., K.M. and G.N. contributed to the paper writing and rewriting.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Machine Intelligence thanks Alex Morehead and Jason Yim for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Note 1, Figs. 1–6 and Tables 1 and 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stebliankin, V., Shirali, A., Baral, P. et al. Evaluating protein binding interfaces with transformer networks. Nat Mach Intell 5, 1042–1053 (2023). https://doi.org/10.1038/s42256-023-00715-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42256-023-00715-4