Abstract
Introduction
The present study examined the gender-specific prognostic value of blood pressure (BP) and its variability in the prediction of dementia risk and developed a score system for risk stratification.
Materials and Methods
This was a retrospective, observational population-based cohort study of patients admitted to government-funded family medicine clinics in Hong Kong between January 1, 2000 and March 31, 2002 with at least 3 blood pressure measurements. Gender-specific risk scores for dementia were developed and tested.
Results
The study consisted of 74 855 patients, of whom 3550 patients (incidence rate: 4.74%) developed dementia over a median follow-up of 112 months (IQR= [59.8–168]). Nonlinear associations between diastolic/systolic BP measurements and the time to dementia presentation were identified. Gender-specific dichotomized clinical scores were developed for males (age, hypertension, diastolic and systolic BP and their measures of variability) and females (age, prior cardiovascular, respiratory, gastrointestinal diseases, diabetes mellitus, hypertension, stroke, mean corpuscular volume, monocyte, neutrophil, urea, creatinine, diastolic and systolic BP and their measures of variability). They showed high predictive strengths for both male (hazard ratio [HR]: 12.83, 95% confidence interval [CI]: 11.15–14.33, P value < .0001) and female patients (HR: 26.56, 95% CI: 14.44–32.86, P value < .0001). The constructed gender-specific scores outperformed the simplified systems without considering BP variability (C-statistic: 0.91 vs 0.82), demonstrating the importance of BP variability in dementia development.
Conclusion
Gender-specific clinical risk scores incorporating BP variability can accurately predict incident dementia and can be applied clinically for early disease detection and optimized patient management.
Keywords: blood pressure variability; risk score; risk stratification, dementia; predictive model
INTRODUCTION
Dementia is a global health concern, particularly in the face of the ageing population and its burden upon healthcare systems. Therefore, predictors for dementia are warranted for early diagnosis and intervention to improve patient prognosis. An increase in both systolic and diastolic blood pressure, below the threshold of hypertension, has been reported to be associated with increased dementia risk.1–3 Moreover, over the past decade, studies have shown that increased blood pressure variability (BPV) was found to be associated with an increased risk of dementia.4–8 However, its clinical application in dementia risk stratification has yet been explored.
Furthermore, studies have reported apparent gender differences in the risk factors for dementia.9–11 Several hypotheses have been raised for the increased dementia risk among women, including the peri- and postmenopausal hormonal changes, difference in apolipoprotein E4 allele inheritance and stronger inflammatory dysregulation.12–15 In addition, gender affects the clinical presentation of dementia, such as a higher frequency of visual hallucination, depression, sarcopenia and frailty among female patients.16–18 However, there is a lack of research on the identification and application of gender-specific dementia risk factors. Therefore, the present study aims to explore the genetic-specific prognostic value of BP and BPV in the prediction of dementia risk and establish clinical risk scores for risk stratification.
MATERIALS AND METHODS
Research design and data
The present cohort consists of patients admitted to government-funded family medicine clinics between January 1, 2000 and March 31, 2002. The patients were identified from the Clinical Data Analysis and Reporting System, a territory-wide database that centralizes patient information from government-funded hospitals in Hong Kong to establish comprehensive medical data, including clinical characteristics, disease diagnosis, laboratory results, and medication prescription details. The system has been previously used by both our team and other teams in Hong Kong.19,20 Data were obtained regarding consecutive patients diagnosed with dementia, excluding those who died or were discharged within 24 hours after the first diastolic/systolic BP measurement and those with less than 3 diastolic/systolic BP measurements (study baseline). Mortality data were obtained from the Hong Kong Death Registry, a population-based official government registry with the registered death records of all Hong Kong citizens. Data on the clinical characteristics, disease diagnosis, laboratory results (including complete blood counts, biochemical tests, and diastolic/systolic BP measurements), and medication prescription details were extracted. Dementia was identified with codes from the International Classification of Disease, Ninth Edition (ICD-9): 331.82, 290.0, 290.1, 290.11, 290.12, 290.13, 290.2, 290.21, 290.3, 290.4, 290.41, 290.42, 290.43, 290.8, 290.9, 294.2, 294.1, 294.11, 294.21, 46.1, 42.0, 294.29. The ICD-9 codes for past comorbidities and historical medication prescriptions are detailed in Supplementary Tables 1 and 2.
Statistical analysis and primary outcomes
The primary outcome was the development of dementia from the study baseline in a time-to-event analysis. Patients were followed up from their admission date until December 31, 2019. We extracted the baseline/latest/maximum/minimum values of diastolic and systolic BP and calculated the temporal variability measures of diastolic and systolic BP:21,22 1) mean, 2) median, 3) standard deviation (SD), 4) root mean square (RMS) by first squaring all blood pressure values then performing square root of the mean of the squares, 5) coefficient of variation (CV) by dividing the BP SD by the mean BP then multiplying by 100, and 6) a variability score (from 0 [low] to 100 [high]) defined as the number of changes in BP of 5 mmHg or more, that is, 100*(number of absolute BP change of each 2 successive measurements > 5)/number of measurements.
Clinical characteristics were summarized using descriptive statistics. Continuous variables were presented as median (95% confidence interval [CI] or interquartile range [IQR]) while categorical variables were presented as count (%). The Mann-Whitney U test was used to compare continuous variables. The χ2 test with Yates’ correction was used for 2 × 2 contingency data, and Pearson’s χ2 test was used for contingency data for variables with more than 2 categories. Univariate Cox regression models were conducted based on male and female subgroups, respectively. Significant univariate predictors of demographics, prior comorbidities, clinical and biochemical tests, medication prescriptions, and BP variabilities were used as input of a multivariate Cox analysis model, adjusted by traditional factors and intercepts. Hazard ratios (HRs) with corresponding 95% CI and P values were reported. All statistical tests were 2-tailed and considered significant if P value <.001. Data analyses were performed using RStudio software (Version: 1.1.456) and Python (Version: 3.6).
RESULTS
Gender-specific cohort clinical characteristics
This retrospective cohort study included 74 855 patients (male = 39.2%). Over the course of follow-up, 3550 patients (incidence rate: 4.74%, including 1287 males and 2263 females) developed dementia after a median follow-up of 112 months (IQR= [59.8–168], max = 242) after initial BP measurement (Supplementary Figure 1). The baseline demographic, biochemical, and clinical parameters are summarized in Table 1 in a gender-specific way. The number of patients in the male cohort was smaller in all age intervals except for [0, 10], [60, 70] and [70, 80] years old. Males more frequently tended to have past comorbidities of cardiovascular diseases (38.81% vs 35.40%, P value < .0001), respiratory diseases (52.55% vs 43.32%, P value < .0001), and renal complications (25.93% vs 16.27%, P value < .0001), but tended less frequently to have diabetes mellitus (13.33% vs 14.48%, P value = .0001) and hypertension (59.05% vs 61.15%, P value = .0043) than females.
Table 1.
Clinical characteristics of male and female patients of the cohort
| Males (N = 29 333, event: 1287) | Females (N = 45 522, event: 2263) | P value | |
|---|---|---|---|
| Median (IQR); Max; N or Count (%) | Median (IQR); Max; N or Count (%) | ||
| Demographics | |||
| Age of first BP test, years | 64.6(51.5–73.2); 99.9; n = 29 333 | 62.3(49.0–72.8); 101.4; n = 45 522 | .1201 |
| [0,10] | 28(0.09%) | 13(0.02%) | .0003*** |
| [10,20] | 287(0.97%) | 322(0.70%) | .0001*** |
| [20,30] | 1307(4.45%) | 1658(3.64%) | <.0001*** |
| [30,40] | 1303(4.44%) | 2644(5.80%) | <.0001*** |
| [40,50] | 3643(12.41%) | 7642(16.78%) | <.0001*** |
| [50,60] | 5133(17.49%) | 8736(19.19%) | <.0001*** |
| [60,70] | 7395(25.21%) | 9804(21.53%) | <.0001*** |
| [70,80] | 7653(26.09%) | 10 275(22.57%) | <.0001*** |
| [80,90] | 1848(6.30%) | 3070(6.74%) | .026* |
| 90+ | 338(1.15%) | 621(1.36%) | .0142* |
|
| |||
| Past comorbidities | |||
| Cardiovascular | 11 387(38.81%) | 16 115(35.40%) | <.0001*** |
| Respiratory | 15 417(52.55%) | 19 721(43.32%) | <.0001*** |
| Renal | 7608(25.93%) | 7408(16.27%) | <.0001*** |
| Endocrine | 1312(4.47%) | 1971(4.32%) | .382 |
| Diabetes mellitus | 3912(13.33%) | 6595(14.48%) | .0001*** |
| Hypertension | 17 322(59.05%) | 27 840(61.15%) | .0043** |
| Gastrointestinal | 11 433(38.97%) | 17 909(39.34%) | .5139 |
| Stroke | 54(0.18%) | 85(0.18%) | .9956 |
|
| |||
| Medications | |||
| ACEI | 5229(17.82%) | 6353(13.95%) | <.0001*** |
| ARB | 136(0.46%) | 277(0.60%) | .0109* |
| Calcium channel blockers | 8675(29.57%) | 11 657(25.60%) | <.0001*** |
| Beta blockers | 7457(25.42%) | 11 316(24.85%) | .1819 |
| Diuretics for heart failure | 1478(5.03%) | 1955(4.29%) | <.0001*** |
| Diuretics for hypertension | 3505(11.94%) | 6184(13.58%) | <.0001*** |
| Nitrates | 3498(11.92%) | 4708(10.34%) | <.0001*** |
| Antihypertensive drugs | 5141(17.52%) | 2804(6.15%) | <.0001*** |
| Anti-Diabetic drugs | 3305(11.26%) | 4893(10.74%) | .0484* |
| Statins and fibrates | 3518(11.99%) | 5718(12.56%) | .0427* |
|
| |||
| Complete blood count tests | |||
| Mean corpuscular volume, fL | 90.8(87.5–94.0); 132.3; n = 11 927 | 89.5(85.9–92.5); 133.0; n = 18 776 | .8711 |
| Basophil, x10^9/L | 0.02(0.01–0.03); 0.6; n = 5397 | 0.02(0.01–0.02); 0.5; n = 7871 | .8921 |
| Eosinophil, x10^9/L | 0.1(0.1–0.295); 9.25; n = 6390 | 0.1(0.1–0.2); 8.8; n = 9454 | .9324 |
| Lymphocyte, x10^9/L | 1.6(1.1–2.15); 137.94; n = 6462 | 1.8(1.3–2.3); 85.28; n = 9572 | .4514 |
| Metamyelocyte, x10^9/L | 0.15(0.1–0.4); 3.0; n = 71 | 0.16(0.08–0.38); 3.0; n = 73 | .831 |
| Monocyte, x10^9/L | 0.5(0.4–0.7); 3.7; n = 6434 | 0.5(0.36–0.6); 6.09; n = 9530 | .9419 |
| Neutrophil, x10^9/L | 4.8(3.61–6.9); 72.38; n = 6431 | 4.4(3.3–6.2); 40.5; n = 9513 | .3612 |
| White blood count, x10^9/L | 7.5(6.13–9.36); 145.2; n = 11 978 | 7.1(5.8–8.8); 6100.0; n = 18 851 | .1782 |
| Mean cell haemoglobin, pg | 30.9(29.6–32.0); 44.1; n = 11 927 | 30.4(29.0–31.5); 46.6; n = 18 775 | .9056 |
| Myelocyte, x10^9/L | 0.18(0.105–0.45); 1.62; n = 67 | 0.17(0.09–0.38); 3.95; n = 76 | .8561 |
| Platelet, x10^9/L | 223.0(184.0–268.0); 1020.0; n = 11 977 | 244.0(203.0–290.5); 1745.0; n = 18 846 | <.0001*** |
| Reticulocyte, x10^9/L | 55.08(35.3–80.5); 324.0; n = 429 | 55.4(39.2–80.8); 460.0; n = 639 | .9122 |
| Red blood count, x10^12/L | 4.61(4.2–4.99); 7.95; n = 11 912 | 4.27(3.95–4.58); 7.08; n = 18 763 | .8967 |
| Hematocrit, L/L | 0.41(0.38–0.44); 0.61; n = 10 669 | 0.38(0.35–0.4); 0.561; n = 17 300 | .7671 |
|
| |||
| Biochemical tests | |||
| K/Potassium, mmol/L | 4.2(3.9–4.5); 10.0; n = 17 388 | 4.2(3.81–4.5); 13.3; n = 25 177 | .9176 |
| Urate, mmol/L | 0.42(0.343–0.5); 1.12; n = 5009 | 0.35(0.28–0.431); 1.395; n = 6173 | .5651 |
| Albumin, g/L | 41.5(39.0–44.0); 58.0; n = 14 593 | 41.2(39.0–43.6); 58.0; n = 21 232 | .9165 |
| Na/Sodium, mmol/L | 140.0(138.2–142.0); 166.09; n = 17 431 | 141.0(139.0–142.0); 181.0; n = 25 240 | .9249 |
| Urea, mmol/L | 6.0(5.0–7.3); 60.9; n = 17 411 | 5.5(4.5–6.8); 53.4; n = 25 207 | .0145* |
| Protein, g/L | 73.1(70.0–77.0); 112.0; n = 14 523 | 74.0(71.0–78.0); 147.0; n = 21 127 | .8934 |
| Creatinine, umol/L | 99.0(88.0–113.0); 1957.0; n = 17 525 | 77.0(68.0–89.0); 1274.0; n = 25 396 | <.0001*** |
| Alkaline Phosphatase, U/L | 78.0(65.0–95.0); 3275.0; n = 12 528 | 78.0(63.0–96.0); 4280.0; n = 18 090 | .9123 |
| Aspartate Transaminase, U/L | 22.0(18.0–30.0); 5110.0; n = 3642 | 21.0(17.0–27.0); 2148.0; n = 5229 | .4564 |
| Alanine Transaminase, U/L | 22.0(16.0–33.0); 3909.0; n = 10 498 | 18.0(13.0–26.0); 1576.0; n = 15 831 | .0023** |
| Bilirubin, umol/L | 10.2(7.9–14.0); 608.0; n = 12 667 | 9.0(6.6–12.0); 669.0; n = 18 274 | .1562 |
| Diabetes mellitus and lipid tests | |||
| Triglyceride, mmol/mol | 1.44(1.0–2.08); 25.77; n = 8635 | 1.41(1.01–2.04); 30.3; n = 12 504 | .8926 |
| LDL, mmol/mol | 3.2(2.6–3.8); 7.92; n = 6359 | 3.3(2.7–3.9); 9.42; n = 8969 | .6721 |
| HDL, mmol/mol | 1.18(1.01–1.39); 4.14; n = 6652 | 1.37(1.16–1.63); 3.29; n = 9338 | .0104* |
| HbA1c, g/dL | 13.6(11.5–14.8); 19.5; n = 10 501 | 12.5(11.1–13.4); 18.1; n = 16 601 | .1551 |
| Cholesterol, mmol/L | 5.13(4.5–5.8); 13.03; n = 8698 | 5.4(4.7–6.09); 13.84; n = 12 597 | .8723 |
| Glucose, mmol/L | 6.0(5.2–7.6); 72.5; n = 12 819 | 5.8(5.1–7.5); 54.3; n = 18 668 | .751 |
|
| |||
| Diastolic blood pressure measures | |||
| Number of tests | 7(5–12); 31; n = 29 333 | 7(6–11); 35; n = 45 522 | .9012 |
| Baseline, mm Hg | 74(69–85); 140.0; n = 29 333 | 79(65–89); 137.0; n = 45522 | .1923 |
| Latest, mm Hg | 73(66–81); 140.0; n = 29 333 | 70(63–79); 144.0; n = 45522 | .8723 |
| Maximum, mm Hg | 82(78–94); 150.0; n = 29 333 | 89(75–98); 144.0; n = 45522 | .0145* |
| Minimal, mm Hg | 65(57–73); 140.0; n = 29 333 | 61(54–70); 128.0; n = 45522 | .1261 |
| Mean, mm Hg | 75(69–81); 140.0; n = 29 333 | 72(66.3–78); 128.0; n = 45 522 | .7862 |
| Median, mm Hg | 75(69–81); 140.0; n = 29 333 | 72(66–78); 128.0; n = 45522 | .4523 |
| Variance | 53.8(31.62–84.52); 882.0; n = 23 964 | 56.6 (32.9–85.2); 1152.0; n = 37 682 | .5621 |
| SD | 7.3(5.6–9.2); 29.7; n = 23 964 | 7.5 (5.7–9.2); 33.9; n = 37 682 | .8723 |
| RMS | 75.4(69.4–81.3); 140.0; n = 29 333 | 72.4(66.7–78.3); 128.0; n = 45 522 | .6778 |
| CV | 0.09(0.07–0.13); 0.33; n = 23 964 | 0.099(0.07–0.12); 0.4; n = 37 682 | .9561 |
| Variability score | 55.2(45.5–66.7); 94.12; n = 23 964 | 56.25(47.76–66.67); 95.46; n = 37 682 | .6241 |
|
| |||
| Systolic blood pressure measures | |||
| Number of tests | 7(5–12); 33; n = 29333 | 8(5–11); 34; n = 45522 | .8923 |
| Baseline, mm Hg | 131(123–152); 244.0; n = 29 333 | 139(120–159); 251.0; n = 45 522 | .0132* |
| Latest, mm Hg | 133(121–146); 237.0; n = 29 333 | 135(120–146); 261.0; n = 45 522 | .2173 |
| Maximum, mm Hg | 156(140–170); 249.0; n = 29 333 | 157(138–173); 274.0; n = 45 522 | .7671 |
| Minimal, mm Hg | 117(106–130); 237.0; n = 29 333 | 114(104–128); 242.0; n = 45 522 | .8921 |
| Mean, mm Hg | 135.96(126.5–145.5); 237.0; n = 29 333 | 135.4(125–145); 242.0; n = 45 522 | .9016 |
| Median, mm Hg | 135.5(126–145.5); 237.0; n = 29 333 | 135(124–145); 242.0; n = 45 522 | .9156 |
| Variance | 165.7(94.4–272.2); 4133.3; n = 23 964 | 167.7(97.0–271.4); 5618.0; n = 37 682 | .8723 |
| SD | 12.9(9.7–16.5); 64.3; n = 23 964 | 12.95(9.9–16.5); 74.95; n = 37 682 | .8912 |
| RMS | 136.5(127.0–146.1); 237.0; n = 29 333 | 136.0(125.3–145.6); 242.0; n = 45 522 | .9015 |
| CV | 0.09(0.07–0.1); 0.3; n = 23 964 | 0.09(0.07–0.11); 0.35; n = 37 682 | .9156 |
| Variability score | 69.2(55.7–77.8); 96.7; n = 23 964 | 70.0(57.1–77.8); 96.97; n = 37 682 | .8954 |
P ≤ .05,
P ≤ .01,
P ≤ .001
In addition, males were more frequently prescribed for angiotensin-converting enzyme inhibitor (ACEI) (17.82% vs 13.95%, P value < .0001), calcium channel blockers (29.57% vs 25.60%, P value < .0001), diuretics for heart failure (5.03% vs 4.29%, P value < .0001), nitrates (11.92% vs 10.34%, P value < .0001), antihypertensive drugs (17.52% vs 6.15%, P value < .0001), and anti-diabetic drugs (11.26% vs 10.74%, P value = .0484), but were less frequently prescribed angiotensin receptor blocker (ARB) (0.46% vs 0.60%, P value = .0109), diuretics for hypertension (11.94% vs 13.58%, P value < .0001), and statins and fibrates (11.99% vs 12.56%, P value = .0427).
Males had lower platelet levels (median: 223 x10^9/L, IQR: 184.0–268, max: 1020 x10^9/L vs 244 x10^9/L, IQR: 203.0–290.5, max: 1745 x10^9/L, P value < .0001), high density lipoprotein (HDL) (median: 1.18 mmol/mol, IQR: 1.01–1.39, max: 4.14 vs median: 1.37 mmol/mol, IQR: 1.16–1.63, max: 3.29 mmol/mol, P value = .0104), maximum of diastolic BP (median: 82 mm Hg, IQR: 78–94, max: 150 mm Hg vs median: 89 mm Hg, IQR: 75–98, max: 144, P value = .0145), and baseline value of systolic BP (median: 131 mm Hg, IQR: 123–152, max: 244 vs median: 139 mm Hg, IQR: 120–159, max: 251 mm Hg, P value = .0132). However, male patients had higher urea levels (6 mmol/L, IQR: 5.0–7.3, max: 60.9 mmol/L vs 5.5 mmol/L, IQR: 4.5–6.8, max: 53.4 mmol/L, P value = .0145), creatinine (median: 99 umol/L, IQR: 88–113, max: 1957 vs 77 umol/L, IQR: 68.0–89, max: 1274 umol/L, P value < .0001), alanine transaminase (median: 22 U/L, IQR: 16.0–33, max: 3909 U/L vs 18 U/L, IQR: 13–26, max: 1576 U/L, P value = .0023),
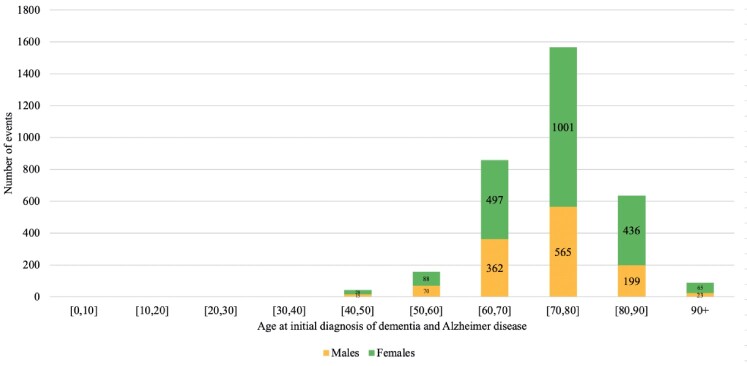
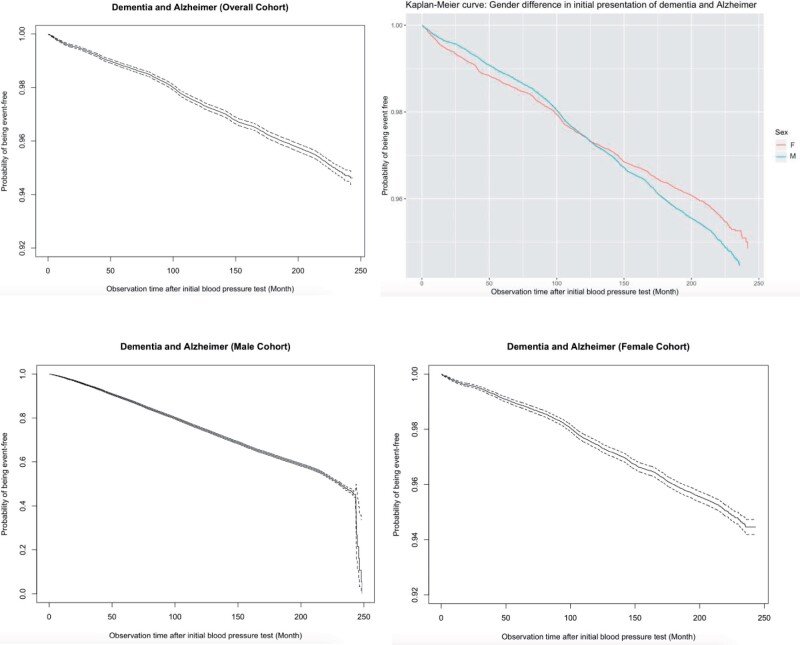
Endocrine (median age= 73.9, IQR = [63.4–82.2]) and gastrointestinal (median age= 74.5, IQR = [63.6, 82.7]) comorbidities, in addition to diabetes mellitus (median age= 75.6, IQR = [66.4, 83.3]), were the 3 earliest comorbidities that occurred prior to dementia, with no significant gender differences (Supplementary Table 3). The incidence rates of female patients were significantly higher than those of male patients in the following age groups of [40, 50–90], and 90+ (Figure 1). The breakdown of incidences with respect to gender and age are shown in Supplementary Table 4, and the baseline characteristics of the dementia subgroup are shown in Supplementary Table 5. Kaplan-Meier curves for the nondementia patients are shown in Figure 2, while those for all-cause mortality are detailed in Supplementary Figure 2.
Figure 1.
Age-specific incidence of dementia diseases between male patients and female patients.
Figure 2.
Survival curves of dementia outcome in the overall cohort, male cohort, and female cohort.
Significant risk predictors of dementia and associations of BP measurements with time-to-dementia
Univariate predictors for incident dementia are summarized in Table 2, while those for mortality among those with dementia are detailed in the Supplementary Table 6. With identified significant univariate predictors as inputs, the following parameters were found to be significant multivariate predictors (Table 3): (1) age of first BP measurement: 40–50 (HR: 1.05, 95% CI: [1.01, 1.26], P < .001), 50–60 (HR: 1.17, 95% CI: [1.06, 1.45], P < .001), 60–70 (HR: 1.43, 95% CI: [1.20, 1.93], P: .001), 70–80 (HR: 1.45, 95% CI: [1.36, 1.93], P < .0001), 80–90 (HR: 1.47, 95% CI: [1.09, 3.06], P < .0001); (2) comorbidities: cardiovascular (HR: 1.10, 95% CI: [1.08, 1.55], P < .0001), respiratory (HR: 1.56, 95% CI: [1.05, 2.31], P: .028), hypertension (HR: 1.21, 95% CI: [1.09, 1.46], P < .0001), gastrointestinal (HR: 1.66, 95% CI: [1.23, 2.23], P: .001); (3) medication: calcium channel blockers (HR: 1.15, 95% CI: [1.04, 1.57], P < .0001), diuretics for hypertension (HR: 1.01, 95% CI: [1.01, 1.44], P < .0001); (4) laboratory parameters: eosinophil count (HR: 0.28, 95% CI: [0.10, 0.77], P: .014), neutrophil count (HR: 1.03, 95% CI: [1.08, 1.47], P < .0001), urate (HR: 0.14, 95% CI: [0.04, 0.53], P: .004), aspartate transaminase (HR: 0.99, 95% CI: [0.97, 1.00], P: .017); (5) diastolic BP: baseline (HR: 1.02, 95% CI: [1.01, 1.21], P < .0001), mean (HR: 1.25, 95% CI: [1.14, 1.57], P < .0001), variance (HR: 1.40, 95% CI: [1.04, 1.51], P < .0001), CV (HR: 1.31, 95% CI: [1.02, 1.65], P < .0001), variability score (HR: 1.22, 95% CI: [1.09, 2.11], P < .0001); (6) systolic BP: baseline (HR: 1.02, 95% CI: [1.01, 1.21] P < .0001), maximum (HR: 1.40, 95% CI: [1.18, 1.42], P < .0001), mean (HR: 1.27, 95% CI: [1.17, 1.61], P < .0001), SD (HR: 1.18, 95% CI: [1.01, 1.69], P < .0001), variability score (HR: 1.43, 95% CI: [1.18, 1.91], P < .0001). Nonlinear relationships between systolic or diastolic BP measurements and the time-to-dementia are shown in Supplementary Figures 3 and 4, respectively.
Table 2.
Univariate predictors of dementia diseases for all patients, males, and females
| All patients | P value | Males | P value | Females | P value | |
|---|---|---|---|---|---|---|
| HR [95% CI] | HR [95% CI] | HR [95% CI] | ||||
| Demographics | ||||||
| Male gender | 0.88[0.83,0.95] | .0005*** | – | – | – | – |
| Age, years | ||||||
| [30,40] | 0.02[0.01, 0.05] | <.0001*** | 0.016[0.002, 0.12] | <.0001*** | 0.014[0.004, 0.06] | <.0001*** |
| [40,50] | 0.07[0.05,0.091] | <.0001*** | 0.08[0.05, 0.14] | <.0001*** | 0.06[0.04, 0.09] | <.0001*** |
| [50,60] | 0.199[0.17, 0.23] | <.0001*** | 0.27[0.21, 0.34] | <.0001*** | 0.17[0.1, 0.2] | <.0001*** |
| [60,70] | 1.2[1.1, 1.8] | <.0001*** | 1.15[1.02, 1.3] | <.0001*** | 1.4[1.1, 2.1] | <.0001*** |
| [70,80] | 2.6[2.4, 2.8] | <.0001*** | 2.3[2.02, 2.5] | <.0001*** | 3.3[2.6, 4.2] | <.0001*** |
| [80,90] | 3.3[3.1, 3.6] | <.0001*** | 2.9[2.5, 3.4] | <.0001*** | 4.6[3.2, 5.0] | <.0001*** |
| 90+ | 2.1[1.7, 2.59] | <.0001*** | 1.7[1.1, 2.5] | .0169* | 3.1[1.8, 4.1] | <.0001*** |
|
| ||||||
| Comorbidities | ||||||
| Cardiovascular | 1.9[1.8,2.1] | <.0001*** | 1.5[1.4,1.7] | <.0001*** | 2.2[2.0,2.4] | <.0001*** |
| Respiratory | 3.8[3.5,4.1] | <.0001*** | 4.2[3.7,4.9] | <.0001*** | 3.8[3.4,4.1] | <.0001*** |
| Renal | 1.6[1.5,1.7] | <.0001*** | 1.4[1.2,1.6] | <.0001*** | 1.8[1.6,2.0] | <.0001*** |
| Endocrine | 0.7[0.6,0.8] | .0002*** | 0.6[0.5, 0.9] | .005** | 0.7[0.6,0.9] | .0116* |
| Diabetes mellitus | 1.3[1.2,1.4] | <.0001*** | 1.1[0.9,1.2] | .452 | 1.4[1.2,1.5] | <.0001*** |
| Hypertension | 1.7[1.6,1.9] | <.0001*** | 1.6[1.4,1.8] | <.0001*** | 1.8[1.7,2.2] | <.0001*** |
| Gastrointestinal | 1.6[1.5,1.8] | <.0001*** | 1.5[1.4,1.7] | <.0001*** | 1.7[1.6,1.9] | <.0001*** |
| Stroke | 1.9[1.8,2.0] | <.0001*** | 1.6[1.4,1.8] | <.0001*** | 2.2[2.0,2.3] | <.0001*** |
|
| ||||||
| Medications | ||||||
| ACEI | 1.3[1.2,1.5] | <.0001*** | 1.1[0.9,1.2] | .362 | 1.6[1.4,1.7] | <.0001*** |
| ARB | 1.1[0.7,1.7] | .687 | 1.3[0.7,2.7] | .427 | 1.0[0.6,1.7] | .934 |
| Calcium channel blockers | 1.4[1.3,1.5] | <.0001*** | 1.2[1.1,1.3] | .004** | 1.6[1.4,1.7] | <.0001*** |
| Beta blockers | 1.1[1.0, 1.2] | .0036** | 1.0[0.9,1.13] | 0.94 | 1.2[1.1,1.3] | .0002*** |
| Diuretics for heart failure | 1.9[1.7, 2.1] | <.0001*** | 1.7[1.4,2.1] | <.0001*** | 2.0[1.7,2.3] | <.0001*** |
| Diuretics for hypertension | 1.3[1.2,1.4] | <.0001*** | 1.08[0.9,1.3] | .335 | 1.4[1.2,1.5] | <.0001*** |
| Nitrates | 1.7[1.5,1.8] | <.0001*** | 1.4[1.2,1.6] | <.0001*** | 1.9[1.7,2.1] | <.0001*** |
| Antihypertensive drugs | 1.6[1.5,1.7] | <.0001*** | 1.8[1.6,2.0] | <.0001*** | 1.6[1.4,1.8] | <.0001*** |
| Antidiabetic drugs | 1.2[1.1,1.4] | <.0001*** | 1.1[0.9,1.3] | .377 | 1.3[1.2,1.5] | <.0001*** |
| Statins and fibrates | 1.1[0.99,1.2] | .0516. | 0.9[0.8,1.1] | .294 | 1.2[1.1,1.4] | .002** |
|
| ||||||
| Complete blood count tests | ||||||
| Mean corpuscular volume, fL | 1.02[1.01,1.03] | <.0001*** | 1.01[0.99,1.02] | .0983. | 1.03[1.02,1.03] | <.0001*** |
| Basophil, x10^9/L | 0.4[0.1,1.6] | .186 | 0.26[0.02,2.82] | .266 | 0.49[0.07,3.44] | .47 |
| Eosinophil, x10^9/L | 0.5[0.4,0.8] | .0008*** | 0.5[0.3,0.8] | .0092** | 0.6[0.4,1.01] | .0546. |
| Lymphocyte, x10^9/L | 0.77[0.7,0.8] | <.0001*** | 0.8[0.7,0.9] | .0001*** | 0.75[0.7,0.8] | <.0001*** |
| Metamyelocyte, x10^9/L | 0.9[0.2,4.1] | .919 | 2.2[0.5,10.1] | .324 | 0.3[0.01,7.74] | .474 |
| Monocyte, x10^9/L | 1.5[1.2,1.7] | <.0001*** | 1.1[0.8,1.5] | .545 | 1.7[1.5,2.1] | <.0001*** |
| Neutrophil, x10^9/L | 1.04[1.03,1.06] | <.0001*** | 1.02[1.0,1.04] | .0828. | 1.06[1.04,1.1] | <.0001*** |
| White blood count, x10^9/L | 1.0[0.999,1.001] | .904 | 1.006[0.99,1.03] | .559 | 1.00[0.99,1.001] | .929 |
| Mean cell haemoglobin, pg | 1.04[1.02,1.06] | <.0001*** | 1.02[0.99,1.05] | .11 | 1.05[1.03,1.07] | <.0001*** |
| Myelocyte, x10^9/L | 0.6[0.1,4.7] | 0.662 | 0.001[0.001,12.5] | .564 | 0.8[0.2,3.7] | .731 |
| Platelet, x10^9/L | 0.998[0.997,0.999] | <.0001*** | 0.998[0.997,0.999] | .0014** | 0.998[0.997,0.999] | .0008*** |
| Reticulocyte, x10^9/L | 0.998[0.99,1.004] | .522 | 0.99[0.98,1.001] | .094. | 1.002[0.99,1.01] | .585 |
| Red blood count, x10^12/L | 0.65[0.6,0.69] | <.0001*** | 0.67[0.6,0.74] | <.0001*** | 0.62[0.56,0.68] | <.0001*** |
| Hematocrit, L/L | 0.02[0.01,0.04] | <.0001*** | 0.007[0.002,0.03] | <.0001*** | 0.03[0.007,0.1] | <.0001*** |
|
| ||||||
| Biochemical tests | ||||||
| K/Potassium, mmol/L | 0.78[0.73,0.85] | <.0001*** | 0.78[0.68,0.89] | .0002*** | 0.8[0.72,0.88] | <.0001*** |
| Urate, mmol/L | 0.4[0.2,0.8] | .007** | 0.14[0.04,0.44] | .0009*** | 1.1[0.46,2.52] | .859 |
| Albumin, g/L | 0.94[0.93,0.95] | <.0001*** | 0.94[0.92,0.95] | <.0001*** | 0.94[0.93,0.96] | <.0001*** |
| Na/Sodium, mmol/L | 0.986[0.97,0.998] | .0194* | 0.97[0.95,0.99] | .0015** | 0.99[0.98,1.01] | .389 |
| Urea, mmol/L | 1.05[1.04,1.06] | <.0001*** | 1.02[1.01,1.04] | .0122* | 1.06[1.05,1.08] | <.0001*** |
| Protein, g/L | 0.97[0.97,0.98] | <.0001*** | 0.97[0.963,0.985] | <.0001*** | 0.97[0.96,0.98] | <.0001*** |
| Creatinine, umol/L | 1.001[1.001,1.002] | <.0001*** | 1.001[0.9995,1.002] | .273 | 1.003[1.002,1.003] | <.0001*** |
| Alkaline phosphatase, U/L | 1.001[1,1.001] | .0183* | 1[0.9985,1.001] | .964 | 1.001[1,1.001] | .003** |
| Aspartate transaminase, U/L | 0.999[0.99,1.001] | .852 | 0.999[0.997,1.001] | .53 | 1.001[0.999,1.002] | .389 |
| Alanine transaminase, U/L | 0.987[0.98,0.99] | <.0001*** | 0.98[0.97,0.98] | <.0001*** | 0.99[0.99,1.00] | .0012** |
| Bilirubin, umol/L | 1.001[0.997,1.01] | .735 | 1.001[0.99,1.01] | .774 | 1.002[0.997,1.01] | .474 |
| Diabetes mellitus and lipid tests | ||||||
| Triglyceride, mmol/mol | 0.95[0.9,1.004] | .0687. | 0.82[0.73,0.92] | .0007*** | 1.012[0.95,1.07] | .685 |
| LDL, mmol/mol | 1.04[0.96,1.13] | .322 | 0.9[0.8,1.05] | .185 | 1.11[1.01,1.23] | .039* |
| HDL, mmol/mol | 1.2[1.02,1.5] | .0336* | 1.7[1.2,2.3] | .002** | 0.95[0.74,1.2] | .661 |
| HbA1c, mmol/mol | 0.99[0.98,0.99] | .002** | 0.99[0.97,0.999] | .0371* | 0.98[0.97,0.998] | .03* |
| Cholesterol, mmol/L | 1.02[0.96,1.07] | .58 | 0.92[0.84,1.01] | .0702. | 1.05[0.99,1.12] | .126 |
| Glucose, mmol/L | 1.03[1.02,1.05] | <.0001*** | 1.02[1.002,1.05] | .0322* | 1.04[1.03,1.06] | <.0001*** |
|
| ||||||
| Diastolic blood pressure measurements | ||||||
| Number of tests | 1.07[0.13,1.23] | .8511 | 0.65[0.23,1.42] | .0611 | 1.03[0.54,1.22] | .1801 |
| Baseline, mm Hg | 1.15[1.11,2.34] | <.0001*** | 1.43[1.01,1.76] | <.0001*** | 1.24[1.01,1.93] | <.0001*** |
| Latest, mm Hg | 1.03[1.01,1.12] | <.0001*** | 1.09[1.02,1.13] | .0045** | 0.99[0.8,0.99] | .234 |
| Maximum, mm Hg | 1.21[1.1,1.83] | <.0001*** | 0.98[0.90,0.99] | .2834 | 1.34[1.03,2.12] | <.0001*** |
| Minimal, mm Hg | 0.98[0.94,0.983] | .6523 | 0.97[0.92,0.98] | .0823 | 0.98[0.91,0.99] | .831 |
| Mean, mm Hg | 1.31[1.11,1.85] | <.0001*** | 1.13[1.03,1.45] | <.0001*** | 1.43[1.01,1.76] | <.0001*** |
| Median, mm Hg | 1.53[1.24,3.13] | <.0001*** | 1.23[1.11,2.1] | <.0001*** | 1.13[1.01,1.4] | <.0001*** |
| Variance | 1.003[1.003,1.003] | <.0001*** | 1.002[1.001,1.003] | <.0001*** | 1.003[1.003,1.004] | <.0001*** |
| SD | 1.074[1.062,1.085] | <.0001*** | 1.052[1.034,1.071] | <.0001*** | 1.086[1.072,1.1] | <.0001*** |
| RMS | 0.97[0.92,0.98] | .035* | 0.96[0.93,0.99] | .2341 | 0.99[0.98,0.991] | .8734 |
| CV | 58.7[69.7,194.2] | <.0001*** | 11.5[5.16,19.6] | <.0001*** | 13.8[4.1,39.3] | <.0001*** |
| Variability score | 1.008[1.006,1.01] | <.0001*** | 14.5[6.13,17.9] | <.0001*** | 13.9[4.4,32.1] | <.0001*** |
|
| ||||||
| Systolic blood pressure measurements | ||||||
| Number of tests | 0.87[0.13,1.23] | .2315 | 0.95[0.63,1.02] | .1956 | 0.73[0.34,1.51] | .8523 |
| Baseline, mm Hg | 1.011[1.01,1.012] | <.0001*** | 1.006[1.003,1.009] | <.0001*** | 1.014[1.012,1.015] | <.0001*** |
| Latest, mm Hg | 1.008[1.006,1.01] | <.0001*** | 1.003[1.001,1.006] | .0157* | 1.01[1.008,1.012] | <.0001*** |
| Maximum, mm Hg | 1.011[1.01,1.013] | <.0001*** | 1.008[1.006,1.01] | <.0001*** | 1.013[1.011,1.015] | <.0001*** |
| Minimal, mm Hg | 1.005[1.003,1.007] | <.0001*** | 1.001[0.9978,1.004] | .615 | 1.008[1.005,1.01] | <.0001*** |
| Mean, mm Hg | 1.016[1.014,1.018] | <.0001*** | 1.009[1.006,1.013] | <.0001*** | 1.02[1.018,1.022] | <.0001*** |
| Median, mm Hg | 1.016[1.014,1.018] | <.0001*** | 1.009[1.005,1.012] | <.0001*** | 1.02[1.017,1.022] | <.0001*** |
| Variance | 1.001[1.001,1.001] | <.0001*** | 1.001[1.001,1.001] | <.0001*** | 1.001[1.001,1.001] | <.0001*** |
| SD | 1.052[1.047,1.057] | <.0001*** | 1.042[1.034,1.051] | <.0001*** | 1.057[1.051,1.063] | <.0001*** |
| RMS | 1.017[1.015,1.019] | <0.0001*** | 1.01[1.006,1.013] | <.0001*** | 1.021[1.018,1.023] | <.0001*** |
| CV | 44.4[18.6,105.9] | <.0001*** | 10.5[2.5,44.8] | <.0001*** | 10.3[3.5,30.8] | <.0001*** |
| Variability score | 1.009[1.007,1.012] | <.0001*** | 1.009[1.005,1.013] | <.0001*** | 1.01[1.007,1.012] | <.0001*** |
P ≤ .05,
P ≤ 0.01,
P ≤ .001
Table 3.
Multivariate predictors of dementia diseases for all patients, males, and females
| All patients | P value | Males | P value | Females | P value | |
|---|---|---|---|---|---|---|
| HR [95% CI] | HR [95% CI] | HR [95% CI] | ||||
| Demographics | ||||||
| Male gender | 0.88[0.62, 1.27] | .5051 | – | – | – | – |
| Age | ||||||
| [30,40] | – | – | – | – | 1.05[1.01,1.37] | .0035** |
| [40,50] | 1.05[1.01, 1.26] | .0003 *** | – | – | 1.03[1.01,1.12] | <.0001*** |
| [50,60] | 1.17[1.06, 1.45] | .0004 *** | – | – | 1.09[1.04,1.20] | <.0001*** |
| [60,70] | 1.43[1.20, 1.93] | .0011 ** | 1.23[1.04,1.31] | <.0001*** | 1.42[1.24,1.72] | <.0001*** |
| [70,80] | 1.45[1.36, 1.93] | <.0001*** | – | – | 1.28[1.11,1.81] | <.0001*** |
| [80,90] | 1.47[1.09, 3.06] | <.0001*** | 1.18[1.01,1.52] | <.0001*** | 1.27[1.06,2.11] | <.0001*** |
| 90+ | 1.21[0.41, 3.57] | .7316 | – | – | 1.67[1.15,3.28] | <.0001*** |
|
| ||||||
| Comorbidities | ||||||
| Cardiovascular | 1.10[1.08, 1.55] | <.0001*** | 1.03[0.32, 3.32] | .9629 | 1.07[1.04,1.37] | <.0001*** |
| Respiratory | 1.56[1.05, 2.31] | .0275 * | 1.71[0.48, 6.10] | .4095 | 1.59[1.22,2.07] | .0006*** |
| Renal | 0.84[0.60, 1.18] | .3239 | 2.08[0.76, 5.69] | .156 | 0.79[0.62,1.02] | .0688. |
| Endocrine | 1.26[1.09, 1.71] | .0089 ** | – | – | – | – |
| Diabetes mellitus | 1.27[0.86, 1.87] | .2260 | – | – | 1.48[1.13,1.94] | .0049** |
| Hypertension | 1.21[1.09, 1.46] | <.0001*** | 1.05[1.03, 4.76] | <.0001*** | 1.24[1.15,1.61] | <.0001*** |
| Gastrointestinal | 1.66[1.23, 2.23] | .0009 *** | 2.80[0.99, 7.89] | .0513. | 1.36[1.10,1.67] | .0043** |
| Stroke | 0.95[0.69, 1.31] | .7431 | 1.35[0.44, 4.14] | .6017 | 1.13[1.02,1.43] | <.0001*** |
|
| ||||||
| Medications | ||||||
| ACEI | 0.93[0.66, 1.30] | .6554 | – | – | 0.84[0.65,1.07] | .1618 |
| Calcium channel blockers | 1.15[1.04, 1.57] | <.0001*** | 0.86[0.30, 2.45] | .7769 | 1.21[1.05,1.41] | <.0001*** |
| Beta blockers | 1.06[0.77, 1.46] | .7140 | – | – | 1.04[0.83,1.31] | .7449 |
| Diuretics for heart failure | 0.84[0.54, 1.33] | .4671 | 0.77[0.13, 4.66] | .7772 | 1.23[1.05,1.61] | <.0001*** |
| Diuretics for hypertension | 1.01[1.01, 1.44] | <.0001*** | – | – | 1.18[1.02,1.55] | <0.0001*** |
| Nitrates | 0.75[0.51, 1.11] | .1499 | 0.74[0.24, 2.30] | .6071 | 1.24[1.17,1.45] | <0.0001*** |
| Antihypertensive drugs | 1.06[0.74, 1.50] | .7566 | 2.20[0.76, 6.32] | .1439 | 0.92[0.66,1.29] | .6351 |
| Antidiabetic drugs | 0.94[0.64, 1.39] | .7661 | – | – | 0.96[0.73,1.27] | .7745 |
| Statins and fibrates | – | – | – | – | 0.86[0.66,1.13] | .2883 |
|
| ||||||
| Complete blood count tests | ||||||
| Mean corpuscular volume, fL | 0.98[0.89, 1.07] | .5890 | – | – | 1.21[1.04,1.67] | <.0001*** |
| Eosinophil, x10^9/L | 1.28[1.10, 1.77] | .0138 * | 0.61[0.06, 6.42] | .6803 | – | – |
| Lymphocyte, x10^9/L | 1.03[0.96, 1.11] | .3769 | 1.28[0.59, 2.78] | .5312 | 0.99[0.87,1.12] | .8179 |
| Monocyte, x10^9/L | 0.66[0.36, 1.21] | .1808 | – | – | 1.11[1.07,1.59] | <.0001*** |
| Neutrophil, x10^9/L | 1.03[1.08, 1.47] | <.0001*** | – | – | 1.22[1.09,1.53] | <.0001*** |
| Mean cell haemoglobin, pg | 0.99[0.82, 1.20] | .9334 | – | – | 0.96[0.84,1.10] | .5891 |
| Platelet, x10^9/L | 1.00[1.00, 1.00] | .3739 | 1.00[0.99, 1.01] | .5299 | 1.00[1.00,1.00] | .6566 |
| Red blood count, x10^12/L | 0.53[0.18, 1.57] | .2488 | 0.82[0.22, 3.05] | .7632 | 0.66[0.25,1.69] | .3824 |
| Hematocrit, L/L | – | – | – | – | 35.71[0.00,191.00] | .5199 |
|
| ||||||
| Biochemical tests | ||||||
| K/Potassium, mmol/L | 0.84[0.65, 1.08] | .1703 | 0.58[0.55, 1.24] | .2612 | 0.96[0.79,1.15] | .6261 |
| Urate, mmol/L | 1.14[1.04, 1.53] | .0037 ** | 0.60[0.01, 35.17] | .8035 | – | – |
| Albumin, g/L | 0.99[0.95, 1.03] | .6981 | 0.91[0.77, 1.07] | .2497 | 1.03[0.99,1.06] | .1187 |
| Urea, mmol/L | 0.98[0.91, 1.05] | .4914 | – | – | 1.17[1.03,1.72] | <.0001*** |
| Na/Sodium, mmol/L | – | – | 1.10[0.94, 1.30] | .2317 | – | – |
| Protein, g/L | 1.03[1.00, 1.06] | .0543. | 1.13[1.01, 1.26] | .0623. | 0.99[0.97,1.01] | .4858 |
| Creatinine, umol/L | 1.00[0.99, 1.01] | .9421 | – | – | 1.00[1.00,1.01] | <.0001*** |
| Aspartate transaminase, U/L | 0.99[0.97, 1.00] | .0166* | 0.96[0.91, 1.01] | .0833. | 1.00[1.00,1.00] | .7237 |
| Alanine transaminase, U/L | – | – | – | – | 1.00[1.00,1.00] | .9126 |
| Diabetes mellitus and lipid tests | ||||||
| Triglyceride, mmol/mol | – | – | 1.22[0.66, 2.26] | .5324 | – | – |
| HDL, mmol/mol | – | – | 2.73[0.78, 9.52] | .1161 | – | – |
| Glucose, mmol/L | 1.02[0.99, 1.06] | .2476 | – | – | 1.01[0.97,1.04] | .6423 |
|
| ||||||
| Diastolic blood pressure measurements | ||||||
| Baseline, mm Hg | 1.14[1.07, 1.52] | <.0001*** | 1.15[1.08, 1.43] | <.0001*** | 1.21[1.02,1.21] | <.0001*** |
| Latest, mm Hg | 1.00[0.98, 1.02] | .1834 | 1.06[0.99, 1.12] | .0816. | – | – |
| Maximum, mm Hg | 1.01[0.96, 1.05] | .8364 | – | – | 1.19[1.06,1.92] | <.0001*** |
| Mean, mm Hg | 1.25[1.14, 1.57] | <.0001*** | 0.87[0.65, 1.17] | .366 | 1.32[1.12,1.79] | <.0001*** |
| Median, mm Hg | 1.04[0.96, 1.13] | .3311 | 1.23[1.01, 1.32] | <0.0001*** | 1.02[0.96,1.08] | 0.4816 |
| Variance | 1.4[1.04, 1.51] | <.0001*** | 1.3[1.07, 1.94] | <.0001*** | 1.11[1.01,1.32] | <.0001*** |
| SD | 1.29[0.97, 1.72] | .0800 | 1.52[0.37, 6.16] | .5582 | 0.96[0.79,1.17] | .6825 |
| CV | 1.31[1.02, 1.65] | <.0001*** | 0.00[0.00, 12.00] | .5327 | – | – |
| Variability score | 1.22[1.09, 2.11] | <.0001*** | 1.19[1.05, 1.83] | <.0001*** | 1.22[1.12,2.41] | <.0001*** |
|
| ||||||
| Systolic blood pressure measurements | ||||||
| Baseline, mm Hg | 1.02[1.01, 1.21] | <.0001*** | 1.03[0.99, 1.07] | .1789 | 1.31[1.09,2.34] | <.0001*** |
| Latest, mm Hg | 1.01[1.00, 1.02] | .1029 | – | – | 1.00[1.00,1.01] | .3566 |
| Maximum, mm Hg | 1.40[1.18, 1.42] | <.0001*** | 1.00[0.94, 1.06] | .9429 | 1.02[1.01,1.03] | <.0001*** |
| Minimal, mm Hg | 1.02[0.99, 1.05] | .2281 | – | – | 1.02[1.00,1.05] | .0322* |
| Mean, mm Hg | 1.27[1.17, 1.61] | <.0001*** | 0.00[0.00, 10.33] | .1196 | 0.71[0.27,1.84] | .4817 |
| Median, mm Hg | 1.00[0.95, 1.04] | .9105 | 0.94[0.82, 1.08] | .399 | 1.03[1.00,1.07] | <.0001*** |
| Variance | 1.00[0.99, 1.00] | .1617 | 0.98[0.94, 1.02] | .251 | 1.15[1.01,1.42] | <.0001*** |
| SD | 1.18[1.01, 1.69] | <.0001*** | 1.86[0.48, 7.22] | .3698 | 0.98[0.85,1.12] | .7148 |
| RMS | 3.69[0.98, 13.81] | .0529. | – | – | 1.31[1.11,3.35] | <.0001*** |
| CV | – | – | 0.00[0.00, 12.00] | .2279 | – | – |
| Variability score | 1.43[1.18, 1.91] | <.0001*** | 1.03[0.98, 1.08] | .2033 | 1.32[1.09,2.11] | <.0001*** |
≤ .05,
P ≤ .01,
P ≤ .001
Gender-specific clinical risk score to predict incident dementia
Based on the findings of multivariate Cox regression and cutoff values of significant predictors, excluding predictive post-hoc medication variables, we developed a clinical risk score for early prediction of dementia in male and female patients in Table 4. For both genders, the following common variables were used: age, prior hypertension, baseline, median, variance, and variability score of diastolic blood pressure and systolic blood pressure. For female patients, the following additional variables were included: prior cardiovascular, respiratory and gastrointestinal diseases, hypertension and stroke, and laboratory examinations.
Table 4.
Clinical risk scores for early prediction of dementia diseases in male (left) and female (right) patients
| Clinical Risk Score for Males |
Clinical Risk Score for Females |
||||
|---|---|---|---|---|---|
| Risk factors | Score | Cutoff | Risk factors | Score | Cutoff |
| Age | Age of first BP | ||||
| [60,70] | 1.23 | Present | [30,40] | 1.05 | Present |
| [80,90] | 1.18 | Present | [40,50] | 1.03 | Present |
| Prior hypertension | 1.05 | Present | [50,60] | 1.09 | Present |
| High diastolic BP baseline, mm Hg | 1.15 | 75.5 mm Hg | [60,70] | 1.42 | Present |
| High diastolic BP median, mm Hg | 1.23 | 73.2 mm Hg | [70,80] | 1.28 | Present |
| High diastolic BP variance | 1.3 | 67.4 | [80,90] | 1.27 | Present |
| High diastolic BP variability score | 1.19 | 59.2 | 90+ | 1.67 | Present |
| High systolic BP median, mm Hg | 1.01 | 141.5 mm Hg | Prior cardiovascular | 1.07 | Present |
| High systolic BP variance | 1.01 | 235.4 | Prior respiratory | 1.59 | Present |
| Prior diabetes mellitus | 1.48 | Present | |||
| Prior hypertension | 1.24 | Present | |||
| Prior gastrointestinal | 1.36 | Present | |||
| Prior stroke | 1.13 | Present | |||
| High mean corpuscular volume, fL | 1.21 | 92.4 fL | |||
| High monocyte, x10^9/L | 1.11 | 0.53 x10^9/L | |||
| High neutrophil, x10^9/L | 1.22 | 5.3 x10^9/L | |||
| High urea, mmol/L | 1.17 | 6.5 mmol/L | |||
| High creatinine, umol/L | 1.00 | 102.4 umol/L | |||
| High diastolic BP baseline, mm Hg | 1.21 | 77.2 mm Hg | |||
| High diastolic BP maximum, mm Hg | 1.19 | 79.1 mm Hg | |||
| High diastolic BP mean, mm Hg | 1.32 | 75.5 mm Hg | |||
| High diastolic BP variance | 1.11 | 69.8 | |||
| High diastolic BP variability score | 1.22 | 68.5 | |||
| High systolic BP baseline, mm Hg | 1.31 | 145.2 mm Hg | |||
| High systolic BP maximum, mm Hg | 1.01 | 169.3 mm Hg | |||
| High systolic BP median, mm Hg | 1.03 | 149.5 mm Hg | |||
| High systolic BP variance | 1.15 | 245.1 | |||
| High systolic BP RMS | 1.31 | 149.23 | |||
| High systolic BP variability score | 1.32 | 0.13 | |||
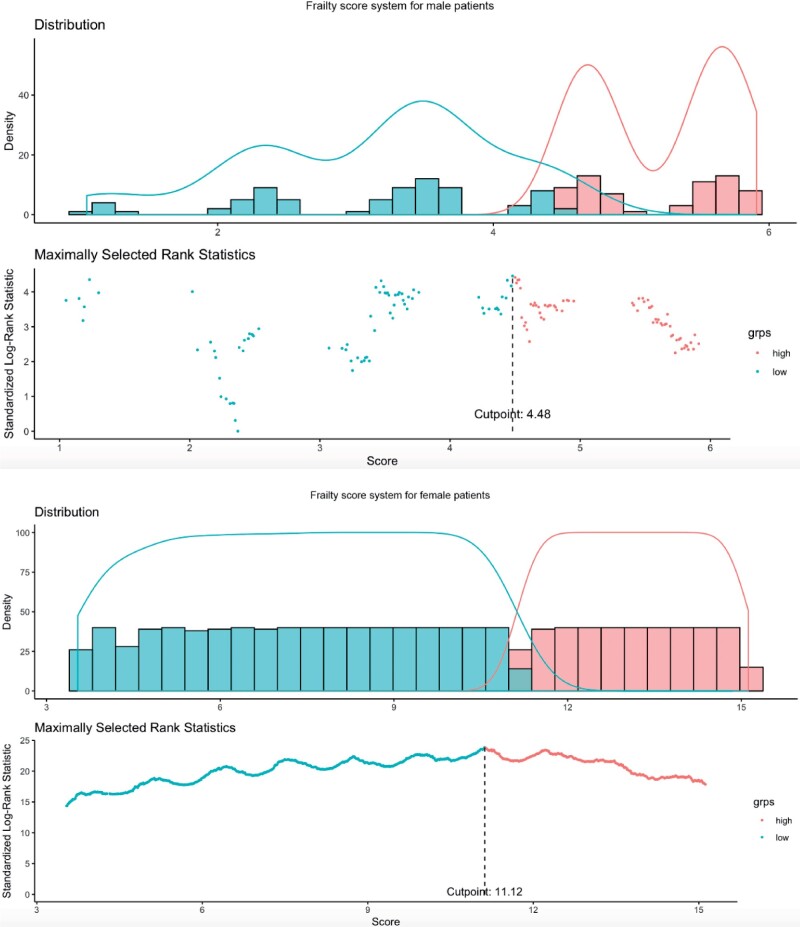
Furthermore, the details of the score for male and female patients with/without dementia are summarized in Supplementary Table 7. Comparing within the gender subgroups, both male (median: 4.22, IQR: 2.36,5.56, max: 9.17 vs median: 3.5, IQR: 2.31,4.77, max: 5.47, P value < .0001) and female (median: 11.58, IQR: 8.82,14.7, max: 26.56 vs median: 8.96, IQR: 6.05,12.22, max: 15.81, P value < .0001) with dementia had a higher score than their nondemented counterparts. The discrimination performance of the scores is shown in Figure 3. For females, the score had a cutoff value of 11.13 and is also able to significantly predict the initial presentation of dementia (HR: 1.13, 95% CI: 1.12–1.24, P value < .0001), and the dichotomized score system shows much more predictive ability (HR: 26.56, 95% CI: 14.44–32.86, P value < .0001).
Figure 3.
Discrimination performance of clinical risk scores for male (top) and female (bottom) patients.
The performance of the scores were compared in Supplementary Table 8 to predict the initial presentation of dementia. For males, the score had a cutoff of 4.48 and can significantly predict initial presentation of dementia (HR: 1.08, 95% CI: 1.05–1.11, P value < .0001), while the dichotomized score system demonstrated even more predictive strength (HR: 12.83, IQR: 11.15–14.33, P value < .0001).
To explore further a simpler score that can be used at baseline (rather than incorporating subsequent results which would not be available at that juncture) (Table 5). In this simplified score, only baseline blood pressure was included. However, the performance metrics (Table 6) showed that there was a reduction in the c-statistic by 0.088 and 0.096 for male and female patients, respectively, indicating the importance of incorporating successive measurements for blood pressure on follow-up to improve risk stratification.
Table 5.
Simplified clinical risk scores for early prediction of dementia diseases in male (left) and female (right) patients after excluding BP variability measures
| Clinical Risk Score for Males |
Clinical Risk Score for Females |
||||
|---|---|---|---|---|---|
| Risk factors | Score | Cutoff | Risk factors | Score | Cutoff |
| Age | Age of first BP | ||||
| [60,70] | 1.33 | Present | [30,40] | 1.04 | Present |
| [80,90] | 1.28 | Present | [40,50] | 1.07 | Present |
| Prior hypertension | 1.05 | Present | [50,60] | 1.06 | Present |
| Lower alanine transaminase, U/L | 0.96 | 23.2 U/L | [60,70] | 1.42 | Present |
| Hematocrit, L/L | 0.23 | 0.45 L/L | [70,80] | 1.31 | Present |
| High diastolic BP baseline, mm Hg | 1.21 | 75.4mm Hg | [80,90] | 1.25 | Present |
| 90+ | 2.15 | Present | |||
| Prior cardiovascular | 1.06 | Present | |||
| Prior respiratory | 1.61 | Present | |||
| Prior diabetes mellitus | 1.52 | Present | |||
| Prior hypertension | 1.43 | Present | |||
| Prior stroke | 1.82 | Present | |||
| High mean corpuscular volume, fL | 1.23 | 94.1 fL | |||
| High monocyte, x10^9/L | 1.19 | 0.53 x10^9/L | |||
| High neutrophil, x10^9/L | 1.24 | 5.2 x10^9/L | |||
| High urea, mmol/L | 1.21 | 6.6 mmol/L | |||
| High diastolic BP baseline, mm Hg | 1.32 | 77.5 mm Hg | |||
| High systolic BP baseline, mm Hg | 1.28 | 143.2 mm Hg | |||
Table 6.
Five-fold cross validation for the comparisons between gender-specific clinical risk scores with BP variabilities and simplified clinical risk scores without BP variabilities for early prediction of dementia diseases
| Systems for males | Cutoff | C-index |
|---|---|---|
| Scoring system considering BP variabilities | 4.48 | 0.9082 |
| Simplified scoring system without BP variabilities | 4.32 | 0.8201 |
| Systems for females | C-index | Cutoff |
| Scoring system considering BP variabilities | 11.12 | 0.9123 |
| Simplified scoring system without BP variabilities | 17.23 | 0.8161 |
DISCUSSION
The main findings of this study include the following:
A combination of clinical, biochemical and systolic/diastolic BP value and variability can be used to predict the onset of dementia;
There are nonlinear associations between diastolic/systolic BP value and variability and the time to dementia manifestation;
A gender-specific, easy-to-use clinical risk score for early prediction of dementia has been constructed and found to be of high predictive strength;
The constructed gender-specific clinical risk scores outperformed the simplified scores that excluded BP variability, demonstrating the importance of the latter in dementia development.
The nonlinear associations between diastolic and systolic BP value and variability reported by the present study support findings from existing studies.7,23–25 There are several hypotheses proposed for the underlying mechanisms of the nonlinear relationship observed. Previous studies propose that the apolipoprotein E4 allele upholds a modulatory role in the effects of BP on cognitive function.26,27 Furthermore, patients with chronic hypertension have been shown to have increased Tau phosphorylation under BP reduction, suggesting that chronic hypertension may increase one’s susceptibility to dementia particularly under extreme BP changes.28,29 Moreover, in a recent study by Walker et al, a pattern of midlife hypertension and late-life hypotension was reported to precede cognitive decline, which suggests a potential early neurological change underlying both the BPV and the cognitive decline. The age-dependent BP change and its associated dementia risk can also be attributed to the nonlinear relationship between BP value and the risk of dementia.
Although it remains controversial whether females have a higher risk for dementia, the presence of gender-specific risk factors has been continuously explored.30,31 First of all, the menopausal transition in middle-aged females was reported to induce a hypometabolic state and can increase brain beta-amyloid deposition thus increasing dementia risk, which is supported by the drastic increase in the HR among the peri- and postmenopausal age groups.32,33 The loss of cardioprotective effect by estrogen among postmenopausal females and resulting BP instability, as reflected by the predictiveness of BPV among female patients, may also underlie their higher risk for vascular dementia.34 In addition, it has been reported that a selective survival of males less susceptible to cardiovascular conditions after mid-life can explain the lower dementia risk among males, which coincides with the presence of cardiovascular comorbidities as a female-only predictor in the present cohort.35 While screening assessments, such as The Montreal Cognitive Assessment, are available for identifying patients with cognitive impairment, carrying out such tests is very time-consuming, and simple clinical scores that can be used to predict longer term dementia development, not just early cognitive impairment, would be helpful for clinicians to manage the patients accordingly.
The plethora of factors underlying the gender differences in dementia risk demonstrates the importance of a gender-specific risk-stratification score system to increase the chances of early disease detection and optimize patient care.
Limitations
Several limitations should be noted for the present study. Given its retrospective and observational nature, this study is prone to selection bias and susceptible to errors due to undercoding and coding errors. Moreover, due to local data availability, only visit-to-visit BP records could be obtained for the analysis of long-term BPV, whereas short-term BPV data were not available. Other important risk factors for dementia, such as the family history of dementia, apolipoprotein E4 allele status, body mass index, and smoking status were not routinely coded into structured data. We have indirectly accounted for the influence of cardiovascular risk factors by examining the prognostic value of cardiovascular comorbidities. In addition, the age distribution for male and female dementia patients were different. For example, the age distribution for female dementia patients was wider. This could potentially explain the need for additional BP measurements for the model development. These scores will be validated in the future when additional data become available.
CONCLUSION
Gender-specific clinical risk scores incorporating BP variability can accurately predict incident dementia and can be applied clinically for early disease detection and optimized patient management.
FUNDING
This work was supported by the National Natural Science Foundation of China (NSFC): 71972164; Health and Medical Research Fund of the Food and Health Bureau of Hong Kong: 16171991; Innovation and Technology Fund of Innovation and Technology Commission of Hong Kong: MHP/081/19; National Key Research and Development Program of China, Ministry of Science and Technology of China: 2019YFE0198600.
AUTHOR CONTRIBUTIONS
JZ, SL: data analysis, data interpretation, statistical analysis, manuscript drafting, critical revision of manuscript
WTW, KBW, KSKL, TTLL, AKCW, TL, CC: project planning, data acquisition, data interpretation, critical revision of manuscript
BMYC: study supervision, data interpretation, statistical analysis, critical revision of manuscript
QZ, GT: study conception, study supervision, project planning, data interpretation, statistical analysis, manuscript drafting, critical revision of manuscript
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
DATA AVAILABILITY STATEMENT
The dataset for this study can be obtained by contacting the corresponding author(s) upon reasonable request for research purposes.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
REFERENCES
- 1. Abell JG, Kivimaki M, Dugravot A, et al. Association between systolic blood pressure and dementia in the Whitehall II cohort study: role of age, duration, and threshold used to define hypertension. Eur Heart J 2018; 39 (33): 3119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gregson J, Qizilbash N, Iwagami M, et al. Blood pressure and risk of dementia and its subtypes: a historical cohort study with long-term follow-up in 2.6 million people. Eur J Neurol 2019; 26 (12): 1479–86. [DOI] [PubMed] [Google Scholar]
- 3. Ding J, Davis-Plourde KL, Sedaghat S, et al. Antihypertensive medications and risk for incident dementia and Alzheimer's disease: a meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol 2020; 19 (1): 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oishi E, Ohara T, Sakata S, et al. Day-to-day blood pressure variability and risk of dementia in a general Japanese elderly population: the Hisayama study. Circulation 2017; 136 (6): 516–25. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Visit-to-visit blood pressure variations: new independent determinants for cognitive function in the elderly at high risk of cardiovascular disease. J Hypertens 2012; 30 (8): 1556–63. [DOI] [PubMed] [Google Scholar]
- 6. Yano Y, Ning H, Allen N, et al. Long-term blood pressure variability throughout young adulthood and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Hypertension 2014; 64 (5): 983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jain S, Kuriakose D, Edelstein I, et al. Right atrial phasic function in heart failure with preserved and reduced ejection fraction. JACC Cardiovasc Imaging 2019; 12 (8 Pt 1): 1460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Heus RAA, Olde Rikkert MGM, Tully PJ, Lawlor BA, Claassen J, Group NS; NILVAD Study Group. Blood pressure variability and progression of clinical Alzheimer disease. Hypertension 2019; 74 (5): 1172–80. [DOI] [PubMed] [Google Scholar]
- 9. Kim S, Kim MJ, Kim S, et al. Gender differences in risk factors for transition from mild cognitive impairment to Alzheimer's disease: a CREDOS study. Compr Psychiatry 2015; 62: 114–22. [DOI] [PubMed] [Google Scholar]
- 10. Choi J, Kwon LN, Lim H, Chun HW. Gender-based analysis of risk factors for dementia using senior cohort. Int J Environ Res Public Health 2020; 17 (19): 7274. doi: 10.3390/ijerph17197274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paul KC, Debes F, Eliasen E, Weihe P, Petersen MS. Incidence, gender influence, and neuropsychological predictors of all cause dementia in the Faroe Islands-the Faroese Septuagenarian cohort. Aging Clin Exp Res 2021; 33 (1): 105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Altmann A, Tian L, Henderson VW, Greicius MD, Alzheimer's Disease Neuroimaging Initiative Investigators. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol 2014; 75 (4): 563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Podcasy JL, Epperson CN. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci 2016; 18 (4): 437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hall JR, Wiechmann AR, Johnson LA, et al. Biomarkers of vascular risk, systemic inflammation, and microvascular pathology and neuropsychiatric symptoms in Alzheimer's disease. J Alzheimers Dis 2013; 35 (2): 363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mosconi L, Berti V, Quinn C, et al. Correction: Perimenopause and emergence of an Alzheimer's bioenergetic phenotype in brain and periphery. PLoS One 2018; 13 (2): e0193314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiu PY, Teng PR, Wei CY, Wang CW, Tsai CT. Gender difference in the association and presentation of visual hallucinations in dementia with Lewy bodies: a cross-sectional study. Int J Geriatr Psychiatry 2018; 33 (1): 193–9. [DOI] [PubMed] [Google Scholar]
- 17. Lee J, Lee KJ, Kim H. Gender differences in behavioral and psychological symptoms of patients with Alzheimer's disease. Asian J Psychiatr 2017; 26: 124–8. [DOI] [PubMed] [Google Scholar]
- 18. Ohta Y, Nomura E, Hatanaka N, et al. Female dominant association of sarcopenia and physical frailty in mild cognitive impairment and Alzheimer's disease. J Clin Neurosci 2019; 70: 96–101. [DOI] [PubMed] [Google Scholar]
- 19. Li CK, Xu Z, Ho J, et al. Association of NPAC score with survival after acute myocardial infarction. Atherosclerosis 2020; 301: 30–6. [DOI] [PubMed] [Google Scholar]
- 20. Ju C, Lai RWC, Li KHC, et al. Comparative cardiovascular risk in users versus non-users of xanthine oxidase inhibitors and febuxostat versus allopurinol users. Rheumatology (Oxford) 2019; 59 (9): 2340–9. [DOI] [PubMed] [Google Scholar]
- 21. Zhou J, Li H, Chang C, et al. The association between blood pressure variability and hip or vertebral fracture risk: a population-based study. Bone 2021; 150: 116015. [DOI] [PubMed] [Google Scholar]
- 22. Zhou J, Lee S, Wong WT, et al. Gender- and age-specific associations of visit-to-visit blood pressure variability with anxiety. Front Cardiovasc Med 2021; 8: 650852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang ZT, Xu W, Wang HF, et al. Blood pressure and the risk of dementia: a dose-response meta-analysis of prospective studies. CNR 2019; 15 (4): 345–58. [DOI] [PubMed] [Google Scholar]
- 24. Rajan KB, Barnes LL, Wilson RS, Weuve J, McAninch EA, Evans DA. Blood pressure and risk of incident Alzheimer's disease dementia by antihypertensive medications and APOE epsilon4 allele. Ann Neurol 2018; 83 (5): 935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walker KA, Sharrett AR, Wu A, et al. Association of midlife to late-life blood pressure patterns with incident dementia. JAMA 2019; 322 (6): 535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of APOE epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA 1999; 282 (1): 40–6. [DOI] [PubMed] [Google Scholar]
- 27. Hofman A, Ott A, Breteler MM, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. Lancet 1997; 349 (9046): 151–4. [DOI] [PubMed] [Google Scholar]
- 28. Glodzik L, Rusinek H, Pirraglia E, et al. Blood pressure decrease correlates with tau pathology and memory decline in hypertensive elderly. Neurobiol Aging 2014; 35 (1): 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Power MC, Tchetgen EJ, Sparrow D, Schwartz J, Weisskopf MG. Blood pressure and cognition: factors that may account for their inconsistent association. Epidemiology 2013; 24 (6): 886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andersen K, Launer LJ, Dewey ME, et al. Gender differences in the incidence of AD and vascular dementia: the EURODEM studies. EURODEM Incidence Research Group. Neurology 1999; 53 (9): 1992–7. [DOI] [PubMed] [Google Scholar]
- 31. Roberts RO, Geda YE, Knopman DS, et al. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic study of aging. Neurology 2012; 78 (5): 342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mosconi L, Berti V, Quinn C, et al. Sex differences in Alzheimer risk: brain imaging of endocrine vs chronologic aging. Neurology 2017; 89 (13): 1382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E. Perimenopause as a neurological transition state. Nat Rev Endocrinol 2015; 11 (7): 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dufouil C, Seshadri S, Chene G. Cardiovascular risk profile in women and dementia. J Alzheimers Dis 2014; 42 (Suppl 4): S353–63. [DOI] [PubMed] [Google Scholar]
- 35. Chene G, Beiser A, Au R, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement 2015; 11 (3): 310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset for this study can be obtained by contacting the corresponding author(s) upon reasonable request for research purposes.