Abstract
Neuropsychological research suggests that “experience-near” semantic memory, meaning knowledge attached to a spatiotemporal or event context, is commonly impaired in individuals who have medial temporal lobe amnesia. It is not known if this impairment extends to remotely acquired experience-near knowledge, which is a question relevant to understanding hippocampal/medial temporal lobe functioning. In the present study, we administered a novel semantic memory task designed to target knowledge associated with remote, “dormant” concepts, in addition to knowledge associated with active concepts, to four individuals with medial temporal lobe amnesia and eight matched controls. We found that the individuals with medial temporal lobe amnesia generated significantly fewer experience-near semantic memories for both remote concepts and active concepts. In comparison, the generation of abstract or “experience-far” knowledge was largely spared in the individuals with medial temporal lobe amnesia, regardless of whether the targets for retrieval were remote or active concepts. We interpret these findings as evidence that the medial temporal lobes may have a sustained role in the retrieval of semantic memories associated with spatiotemporal and event contexts, which are cognitive features often ascribed to episodic memory. These results align with recent theoretical models proposing that the hippocampus/medial temporal lobes support cognitive processes that are involved in, but not exclusive to, episodic memory.
INTRODUCTION
Prior research on human amnesia has found that knowledge acquired in the remote past is largely spared by medial temporal lobe lesions. Individuals with relatively isolated medial temporal lobe amnesia often have normal retrograde semantic memory for a range of information, including vocabulary, world facts, and object names, with impairments usually limited to knowledge acquired in the decade preceding the injury (Moscovitch, Nadel, Winocur, Gilboa, & Rosenbaum, 2006; Verfaellie, Koseff, & Alexander, 2000; Verfaellie, Reiss, & Roth, 1995). New semantic learning in medial temporal lobe amnesia, in contrast, is often slow (Duff, Covington, Hilverman, & Cohen, 2020; Gardiner, Brandt, Baddeley, Vargha-Khadem, & Mishkin, 2008; Manns, Hopkins, & Squire, 2003; Baddeley, Vargha-Khadem, & Mishkin, 2001; Postle & Corkin, 1998), although some knowledge acquisition is possible (Elward & Vargha-Khadem, 2018; O'Kane, Kensinger, & Corkin, 2004; Corkin, 2002; Vargha-Khadem et al., 1997; Tulving, Hayman, & Macdonald, 1991). Researchers have taken this retrograde–anterograde profile to mean that semantic memories may depend on the medial temporal lobes until a stable memory trace is formed in the neocortex, at which point the medial temporal lobes are no longer necessary for retrieval (Winocur & Moscovitch, 2011; Squire & Zola, 1998; Nadel & Moscovitch, 1997).
Recent neuropsychological findings, however, raise doubt about a complete separation of remote semantic memory from the hippocampus/medial temporal lobes. Several studies have found that middle-aged and older adults with medial temporal lobe amnesia have difficulty retrieving detailed stories from fairytales learned as a child, and they struggle to provide elaborative explanations of social issues that took place in remote life periods (Verfaellie, Bousquet, & Keane, 2014; Race, Keane, & Verfaellie, 2013; Rosenbaum, Gilboa, Levine, Winocur, & Moscovitch, 2009). Individuals with medial temporal lobe amnesia also have less elaborative associative semantic networks surrounding remotely acquired concepts (Klooster & Duff, 2015), which may not be completely attributed to compromised knowledge updating since the onset of amnesia (i.e., anterograde amnesia; Klooster, Tranel, & Duff, 2020). Individuals with medial temporal lobe amnesia also may have subtle object naming and generative word use deficits, especially for more visually complex and less familiar concepts (Hilverman & Duff, 2021; Hilverman, Cook, & Duff, 2017; although also see Race, Carlisle, Tejwani, & Verfaellie, 2021). These findings can be interpreted as evidence that certain cognitive processes often viewed as essential to episodic memory, such as generative retrieval, scene construction, visual imagery, and relational processing, may be applied flexibly to other forms of memory and cognition (Duff et al., 2020; Lynch, Keane, & Verfaellie, 2020; Rosenbaum et al., 2009). As a consequence, the hippocampus/medial temporal lobes may have a sustained role in semantic memory, specifically when knowledge retrieval involves these cognitive processes.
Building on this idea, in the present study, we ask whether the medial temporal lobes have a sustained role in the retrieval of semantic memories that are “experience-near” in content. Semantic memories are thought to be stored at various levels of abstraction from prior experiences (Irish & Vatansever, 2020; Renoult, Irish, Moscovitch, & Rugg, 2019; Renoult, Davidson, Palombo, Moscovitch, & Levine, 2012; Conway, 2005; Craik, 2002). At one extreme are “experience-far” semantic memories: knowledge that is devoid of the spatiotemporal and broader event context that accompanied its acquisition (Grilli & Verfaellie, 2014, 2016). Such knowledge is abstract and includes most personality traits and much of what makes up vocabulary knowledge. Examples of experience-far knowledge include knowing that teachers tend to be patient and apples are a type of fruit. At the other extreme are “experience-near” semantics, meaning knowledge that remains partly attached to the spatiotemporal and event contexts of prior experiences (Grilli & Verfaellie, 2016). Such knowledge includes what Neisser referred to as “repisodic” memories (Neisser, 1981) or what Renoult and colleagues call “repeated events” (Renoult et al., 2012). Examples of experience-near knowledge include knowing that lions can be seen in zoos and postal workers leave letters in your mailbox. In these examples, the knowledge that is retrieved is attached to an event or action context (e.g., visiting a zoo, placing letters in a mailbox) and therefore is not completely separated from cognitive processes often theoretically viewed as pivotal to episodic memory.
Initial research involving individuals with medial temporal lobe amnesia appears to support a role for the medial temporal lobes in experience-near, but not experience-far, semantic memory. In a study by Grilli and Verfaellie (Grilli & Verfaellie, 2016), individuals with medial temporal lobe amnesia showed a deficit in the ability to retrieve experience-near personal semantics to support self-defining traits and roles, whereas experience-far personal semantics were normal. Wank and colleagues (Wank et al., 2022) demonstrated that a selective reduction in experience-near personal semantic memory in medial temporal lobe amnesia may be present while describing the life story or elaborating on unique life events. fMRI studies with healthy adults appear to converge with these neuropsychological findings, showing that autobiographical fact retrieval, including experience-near personal knowledge, is associated with the medial temporal lobes as well as areas of the neocortex associated with knowledge storage (Teghil, Bonavita, Procida, Giove, & Boccia, 2022; Martinelli, Sperduti, & Piolino, 2013; Maguire & Frith, 2003). Findings from studies using electroencephalography also suggest that personal semantic memory consists of repeated or experience-near subtypes that have commonalities with the neural signature of episodic memory (Tanguay et al., 2018; Renoult et al., 2015, 2016). Finally, recent research suggests that individuals with Alzheimer's disease dementia, who typically have disproportionate atrophy in the medial temporal lobes, may show a shift toward experience-farness in the semantic memories that they generate while narrating unique events (Strikwerda-Brown, Mothakunnel, Hodges, Piguet, & Irish, 2019).
Despite the apparent connection between experience-near semantic memory and the hippocampus/medial temporal lobes, whether the medial temporal lobes have a sustained role in the retrieval of remotely acquired experience-near semantic memories remains unclear. Critically, prior work on this topic probed personal semantic memories and therefore centered on the self-concept. Although the self-concept is a remotely formed knowledge structure, it may be considered “active,” in the sense that it is likely to have been updated over time (Duff et al., 2020; Klooster & Duff, 2015). Remotely formed active concepts pose a challenge for studying the status of remote memory because we cannot easily determine whether the associated knowledge was acquired in the distant past, or instead acquired or updated through recent experience (Klooster & Duff, 2015). By extension, we do not know if the previously reported amnesia-associated drop in experience-near knowledge is strictly an impairment in the recent acquisition and updating of knowledge about the self. In other words, the experience-near semantic memory impairment may not extend to remote knowledge.
The objective of the present study is to address this methodological issue and test whether remote experience-near semantic memory depends on the medial temporal lobes. To do so, we developed a novel semantic memory task centered on retrieving knowledge related to remotely formed, nonactive concepts. To target nonactive concepts, we focused on occupations that have dropped in visibility in American society, meaning professions that, although popular decades ago, are now rare in the workforce. Take, for example, the milkman. Most middle-aged and older individuals who grew up in America have some knowledge of who the milkman was and what role they served in the workforce. However, the milkman has not had a visible role in American society for decades, nor does the profession commonly appear in the media or contemporary literature. A person's knowledge of the milkman, therefore, was likely acquired remotely and stored in a “dormant” state. As a result, the knowledge that a person associates with professions like the milkman provides a window into the status of remote knowledge that has not had much opportunity to be updated by recent experience.
Here, we examined how individuals with medial temporal lobe amnesia and healthy demographically matched individuals defined a series of occupations that dropped in visibility decades ago. Similar to our prior work (Wank et al., 2022; Grilli & Verfaellie, 2016), we scored participants' answers as either experience-near or experience-far semantic memories. For comparison, we also examined occupations that have maintained a visible presence in society and are therefore active concepts. We hypothesized that if the medial temporal lobes have a necessary and sustained role in the retrieval of the spatiotemporal or event context of experience-near semantic memories, individuals with medial temporal lobe amnesia will not only struggle to retrieve experience-near facts while describing active occupations, but also while describing low-visibility “remote” occupations. We also hypothesized that if the connection between the medial temporal lobes and semantic knowledge is specific to retrieval under conditions invoking episodic memory-associated cognitive processes, the retrieval of experience-far semantic memories will be spared in medial temporal lobe amnesia regardless of concept remoteness.
METHODS
Participants
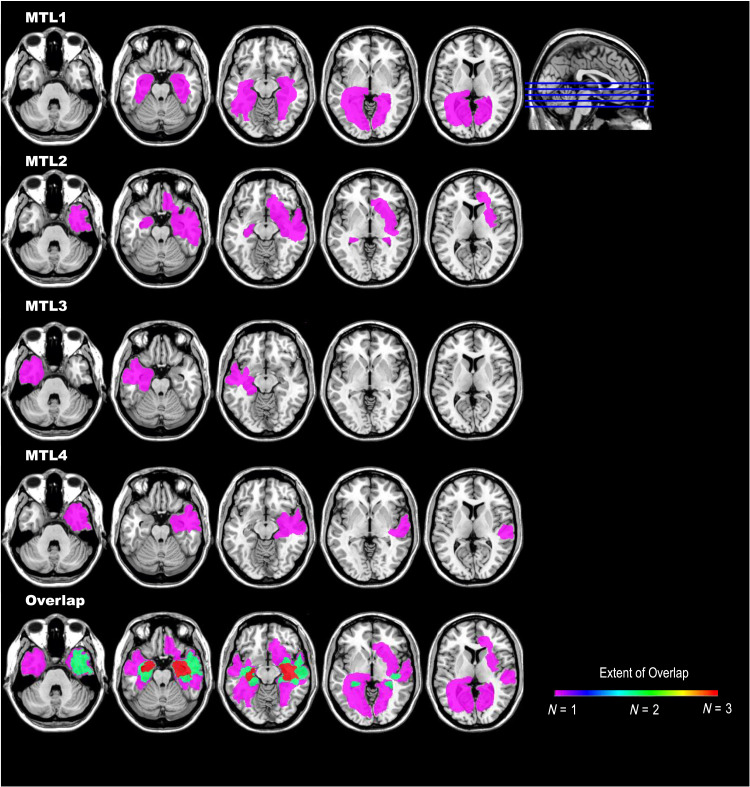
We obtained written informed consent and administered the procedures involved in this study in accordance with the institutional review board at the University of Arizona. We enrolled four individuals with medial temporal lobe amnesia. This included two men and two women ranging in age from 48 to 79 years old at time of testing. These individuals were between 2 and 6 years post medial temporal lobe lesion onset at the time of testing. Each individual had documented impairment in new episodic memory learning, as reported in Table 1. Basic tasks assessing other cognitive domains were largely spared, although there were nonsystematic deficits in working memory, processing speed, and object naming across the individuals. Etiology of amnesia included stroke (MTL1), encephalitis (MTL2), surgical resection for temporal lobe epilepsy (MTL3), and tumor with surgical resection (MTL4). MRI/CT scans confirmed bilateral (MTL1 and MTL2) or unilateral (MTL3 and MTL4) medial temporal lobe lesions. To illustrate the lesions and show areas of overlap among the four individuals, we manually traced their lesions using available MRI/CT scans on the ch2 template in MRICron (Rorden & Brett, 2000). We used anatomical landmarks and multiple slices and angles to identify lesion boundaries for tracing on the template. Figure 1 shows the lesions for each individual along with images showing overlap. As shown in Figure 1, the greatest extent of lesion and areas of overlap are in the medial temporal lobes. As can also be seen in Figure 1, all individuals had lesions extending beyond the medial temporal lobes, although the extramedial temporal lobe lesions were largely non-overlapping.
Table 1. .
Neuropsychological Profiles of Individuals with Amnesia
| MTL1 | MTL2 | MTL3 | MTL4 | |
|---|---|---|---|---|
| WAIS IV | ||||
| Full-Scale IQ | std = 83/Low average | std = 84/Low average | std = 110/Average | std = 89/Low average |
| Verbal Comprehension Index | std = 107/Average | std = 96/Average | std = 98/Average | std = 89/Low average |
| Perceptual Reasoning Index | std = 82/Low average | std = 94/Average | std = 117/High average | std = 92/Average |
| Working Memory Index | std = 89/Low average | std = 74/Impaired | std = 97/Average | std = 100/Average |
| Processing Speed Index | std = 59/Impaired | std = 79/Impaired | std = 122/High average | std = 84/Low average |
| WMS-IV | ||||
| Auditory Memory Index | std = 51/Impaired | std = 60/Impaired | std = 67/Impaired | std = 69/Impaired |
| Visual Memory Index | std = 58/Impaired | std = 44/Impaired | std = 107/Average | std = 69/Impaired |
| Immediate Memory Index | std = 60/Impaired | std = 53/Impaired | std = 91/Average | std = 70/Impaired |
| Delayed Memory Index | std = 43/Impaired | std = 46/Impaired | std = 80/Impaired | std = 61/Impaired |
| Pyramids and Palm Trees (Words) | 92.31%/WNL | 98.08%/WNL | 100%/WNL | 100%/WNL |
| Cambridge Naming Test | z = −11.6/Impaired | z = −1.7/Borderline | z = −0.6/Average | z = 0.6/Average |
WAIS-IV = Wechsler Adult Intelligence Scale, 4th Edition; WMS-IV = Wechsler Memory Scale, 4th Edition; WNL = within normal limits.
Figure 1. .
Lesion location and overlap among the individuals with medial temporal lobe amnesia. As shown here, individuals had damage to bilateral (MTL1 and MTL2) or unilateral (MTL3 and MTL4) hippocampal and surrounding medial temporal lobe structures. Lesions extended into the cortex in all cases, although there was limited overlap. Lesions were manually drawn on the ch2 atlas in MRICron and are shown such that left reflects the left side of the brain. The color bar shows the amount of overlap in lesion location across the four individuals.
We enrolled eight cognitively normal individuals who were selected to match the individuals with medial temporal lobe amnesia on key demographics, p ≥ .30. This included age (mean/std amnesic participants = 59.3/14.0 years, mean/std controls = 59.4/13.2 years), education (mean/std amnesic participants = 16.3/3.5 years, mean/std controls = 15.8/2.3 years), gender (controls = 4 women and 4 men), and verbal intelligence (mean/std amnesic participants = 97.5/7.4, mean/std controls = 102.0/6.4).
Control participants were also matched to the individuals with medial temporal lobe amnesia on familiarity with the occupations examined in the present study. To match on occupation familiarity, we gave the individuals with amnesia a prequestionnaire asking them to rate on a scale from one (very unfamiliar) to four (very familiar) their familiarity with each of the occupations. This not only allowed us to ensure that the individuals with medial temporal lobe amnesia were familiar with the occupations, but we used their data to prescreen potential controls, making sure they closely matched their demographically similar individual with amnesia. Controls had to be an exact match on at least four out of six remote and four out of six active occupations, no more than one point difference on the remaining occupations, and at least somewhat familiar with each occupation (i.e., a score of 3) to be considered a close match to one of the individuals with medial temporal lobe amnesia.
We justified our sample sizes given they were in line with prior research on this topic, which has varied from single-subject case designs to modestly sized group studies (Wank et al., 2022; Grilli & Verfaellie, 2016; Klooster & Duff, 2015; Verfaellie et al., 2014; Rosenbaum et al., 2009).
Semantic Memory Task
The semantic memory task required describing defining features of a series of occupations. Six of the occupations were “remote,” meaning they have significantly dropped in their visible presence, although not all are completely absent from the American workforce. These occupations are milkman, switchboard operator, bowling pin setter, cobbler, soda jerk, and chimney sweep. The other six occupations have been part of the workforce for decades (or more) and remain a highly visible part of society. The active occupations are teacher, firefighter, accountant, store manager, politician, and actor. The 12 occupations were selected from a larger list, based on a prescreening of familiarity among the individuals with medial temporal lobe amnesia. We selected 12 occupations for which all individuals with amnesia reported being somewhat or very familiar with the occupation (i.e., 3 or 4 on a scale from 1–4, from very unfamiliar to very familiar). On a separate day from their familiarity screening, participants were presented the occupations in a quasirandom order, such that no more than two remote or active occupations were presented in sequence. For each occupation, participants were asked to come up with a few defining features of each profession. They were given the example of a medical doctor and told that “When asked to define a medical doctor, some people might say ‘medical doctors are very knowledgeable’ and ‘medical doctors meet you in their office and ask questions about your health’. Many people would consider these defining features of most doctors. Try to explain each feature in one or two sentences. I would like you to tell me your responses and I'll write them down.” Participants were asked to generate up to six defining features per occupation. The experimenter asked, “Can you think of another feature that defines [occupation]?”, after each defining feature was provided (with the exception of the sixth). If a participant reported that they could not think of any more features before six, the experimenter moved on to the next occupation. The task was not timed.
After completing all 12 occupations, the control participants were asked to revisit each occupation one by one and report when they were last exposed to the occupation. Specifically, they were told, “Exposure could come in many forms, including watching a show or reading a book in which you experience a character in that profession, or meeting someone in that profession ‘in real life.’ Alternatively, perhaps you heard someone talk about a person who works in the profession or share a story in which they interacted with someone in that profession. Any exposure is relevant to us, so think broadly about when you might have last been exposed to the profession.” Participants were then given the following response options: past week, past year, 1–5 years ago, 6–10 years ago, 11–20 years ago, and more than 20 years ago. The individuals with amnesia were not asked to rate recent exposure to the occupations because we were concerned about the accuracy of such ratings considering their memory impairment. We also were not confident that informants would be accurate reporters of such information given variability in living situations and closeness between informant and participant.
To prepare the occupation-defining semantic memories for scoring, they were reorganized so that all memories for a single occupation, across all participants, were presented together and in a random order. Consistent with prior work (Wank et al., 2022; Grilli & Verfaellie, 2016), we created a scoring protocol that included definitions and examples of experience-near and experience-far semantic memories. The Data Availability section includes a link to this scoring protocol. Using this scoring protocol, each occupation-defining semantic memory was scored by the first author who was blind to participant status for each response. The first author has extensively trained on scoring experience-near versus experience-far semantic memories and has achieved excellent reliability with independent raters across multiple data sets. To confirm reliability in the current data set, 33% of the occupations (equally drawn from remote and active occupations) were selected from each participant, and each of the semantic memories from these occupations was scored by a second rater, in a blinded fashion. Interrater reliability between the primary and secondary scorer on this subsample was excellent for experience-near and experience-far semantic memories (intraclass correlation coefficients > .92, two-way mixed, single-rater, absolute agreement). Examples of experience-near, occupation-defining semantic memories include “They operated on the roofs of buildings” for a chimney sweep and “They slide down the fire pole, because rooms were usually above the trucks” for a firefighter. Examples of experience-far, occupation-defining semantic memories include “This was mostly a female profession” for a switchboard operator and “They try to shape the law and policies of the country” for a politician.
Statistical Analyses
We used a combination of R and Jamovi to run the statistical analyses, and we used R to create the accompanying figures (R Core Team [2019], https://www.R-project.org/; The Jamovi Project, 2023, retrieved from https://www.jamovi.org). For the control ratings of remoteness, a response of 6–10 years or greater was considered remote exposure, and 5 years or less was considered recent exposure. We then compared the frequency of remote and recent responses within the remote and active occupations using chi-square significance testing. We also compared overall familiarity for remote and active occupations in this same manner. We analyzed the semantic memory data using a 2 (Group: amnesia vs. control) × 2 (Occupation Type: remote vs. active) × 2 (Semantic Type: experience-near vs. experience-far) mixed ANOVA (the assumptions of normality and homogeneity of variance were met). Significant main effects and interactions were followed with planned simple effect analyses. We also used Bayesian analyses to better understand the meaning of null results.
RESULTS
Control participants provided 42 ratings of “remote exposure” and six ratings of “recent exposure” for the remote occupations. For the active occupations, they provided 47 ratings of recent exposure and one rating of remote exposure. The relative frequency of remote versus recent exposure significantly varied for remote and active occupations, in the expected direction, χ2 = 70.81, p < .001. The relative frequency of somewhat and very familiar responses significantly differed for remote and active occupations, such that participants skewed more toward very familiar ratings for active occupations, χ2 = 44.86, p < .001.
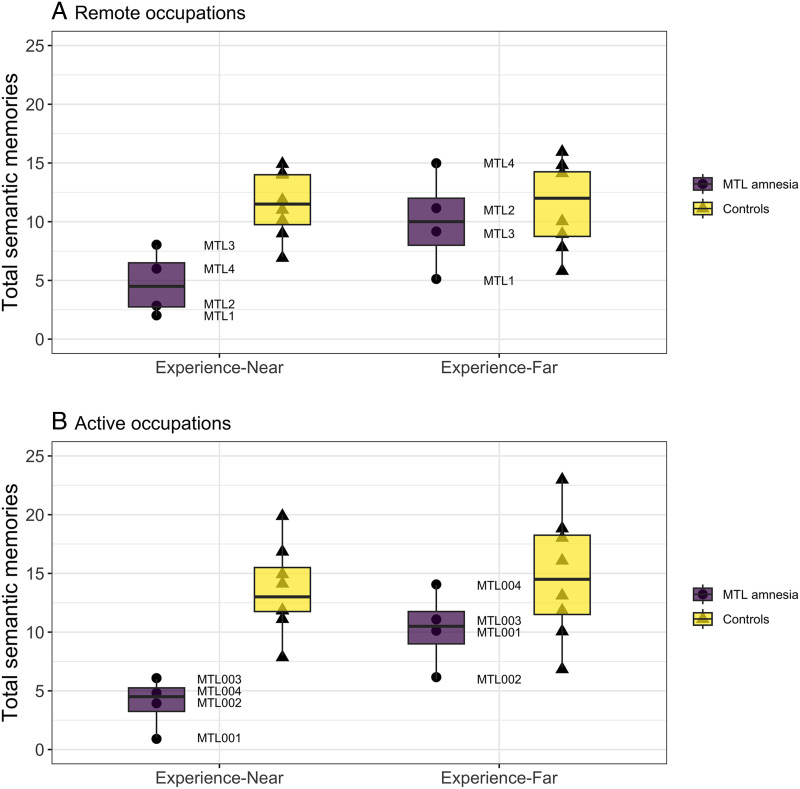
The semantic memory results from the occupations memory task are shown in Figure 2. As shown in Figure 2, for both remote and active occupations, the two individuals with bilateral hippocampal/medial temporal lobe lesions (i.e., MTL1 and MTL2) showed the lowest overall retrieval of experience-near knowledge. The individuals with unilateral lesions were either slightly within or outside the range of experience-near scores from the control participants for remote occupations, and all individuals with medial temporal lobe amnesia scored outside the range of control scores for active occupations. For comparison, three of the individuals with medial temporal lobe amnesia scored within the range of the control participants for experience-far knowledge for either remote or active occupations. MTL1 was slightly outside the experience-far range of control scores for remote occupations, and MTL2 was the same for active occupations.
Figure 2. .
Semantic memories generated for remote and active concepts. Individuals with medial temporal lobe amnesia generated significantly fewer experience-near, occupation-defining facts for remote (A) and active (B) concepts, relative to controls. The generation of experience-far facts, however, did not significantly vary by group for either remote (A) or active (B) concepts. A slight jitter was applied to the data points to improve visibility.
Aligning with these patterns of scores, the 2 (Group: amnesia vs. control) × 2 (Occupation Type: remote vs. active) × 2 (Semantic Type: experience-near vs. experience-far) mixed ANOVA revealed a significant effect of Group such that the individuals with medial temporal lobe amnesia generated fewer semantic memories (mean = 29, SD = 9.87) relative to the control participants (mean = 51.4, SD = 8.65; F(1, 10) = 16.4, p = .002, η2 = .62). There also was a significant effect of Semantic Subcategory Type such that experience-far semantic memories (mean = 24.3, SD = 6.68) were more common than experience-near semantic memories (mean = 19.7, SD = 9.49), F(1, 10) = 8.11, p = .02, partial η2 = .45. There was not a significant effect of Occupation Type (remote occupations: mean = 20.3, SD = 6.30; active occupations: mean = 23.7, SD = 9.29), F(1, 10) = 1.20, p = .30, partial η2 = .11.
There was a significant interaction between Group and Semantic Memory Subcategory Type, F(1, 10) = 5.48, p = .04, η2 = .35. Post hoc tests showed that the individuals with medial temporal lobe amnesia showed a significant disruption of experience-near semantic memories (amnesic group: mean = 8.75, SD = 4.79; control group: mean = 25.1, SD = 5.44), t(10) = 5.09, p < .001, but not experience-far semantic memories (amnesic group: mean = 20.3, SD = 6.18; control group: mean = 26.3, SD = 6.32), t(10) = 1.56, p = .15. Occupation Type did not significantly interact with Group, F(1, 10) = 1.75, p = .22, η2 = .15, or Semantic Subcategory Type, F(1, 10) = 0.32, p = .58, η2 = .03. Finally, there was not a significant three-way interaction between Group, Semantic Memory Subcategory Type, and Occupation Type, F(1, 10) = 0.001, p = .97, η2 = .00.
Although there was not a significant three-way interaction, we followed up with independent-samples t tests comparing groups on both experience-near and experience-far semantic memory for remote and active occupations separately, given the importance of knowing the status of remote memory in the individuals with amnesia. These results confirmed that experience-near semantic memories were significantly lower in the individuals with medial temporal lobe amnesia for remote occupations (mean = 4.75, SD = 2.75) relative to control participants (mean = 11.5, SD = 2.78), t(10) = 3.98, p = .003, d = 2.44, and the individuals with medial temporal lobe amnesia were significantly lower for active occupations (amnesic group: mean = 4.00, SD = 2.16) relative to control participants (mean = 13.6, SD = 3.74), t(10) = 4.7, p < .001, d = 2.88. Experience-far semantic memories, however, were not significantly lower for remote occupations (amnesic group: mean = 10.0, SD = 4.16; control group: mean = 11.5, SD = 3.70), t(10) = 0.64, p = .54, or active occupations (amnesic group: mean = 10.3, SD = 3.30; control group: mean = 14.8, SD = 5.23), t(10) = 1.55, p = .15, in individuals with amnesia compared with controls.
Despite the nonsignificant findings for experience-far semantic memories, numerically, the individuals with amnesia were lower than the control participants. To better contextualize these findings, we followed up with Bayesian independent-samples t tests, which can speak to the degree to which the evidence supports the alternative or null hypothesis. For experience-near semantic memories, the evidence was strong to very strong in favor of an amnesia-associated deficit (BF10 = 13.21 for remote and 30.84 for recent). A follow-up Bayesian mixed ANOVA found very strong evidence for an effect of Group on experience-near semantic memories (BF|inclusion = 54.9), but only anecdotal, or weak, evidence for an interaction between Occupation Type (i.e., remote vs. active occupations) and Group (BF|inclusion = 1.61). For experience-far semantic memories, the evidence was anecdotally in favor of the null hypothesis (BF10 = 0.55 for remote and 0.97 for recent).
Finally, given that there was a main effect of Group on semantic memory generation, we calculated proportional scores for experience-near semantic memories relative to total semantic memories and conducted a 2 (Group: amnesia vs. control) × 2 (Occupation Type: remote vs. active) mixed ANOVA. This revealed a significant effect of Group, F(1, 10) = 16.9, p = .002, partial η2 = .63, such that individuals with amnesia generated a lower proportion of experience-near semantic memories relative to controls (amnesic group: mean = 0.29, SD = 0.10; control group: mean = 0.49, SD = 0.07). There was not, however, a significant effect of Occupation Type (remote occupations: mean = 0.44, SD = 0.14; active occupations: mean = 0.42, SD = 0.15), F(1, 10) = 0.36, p = .56, partial η2 = .04, nor did occupation type significantly interact with group, F(1, 10) = 0.04, p = .85, η2 = .004.
DISCUSSION
Several theories suggest that the medial temporal lobes have a time limited role in the retrieval of semantic memories (Winocur & Moscovitch, 2011; Squire & Zola, 1998; Nadel & Moscovitch, 1997). Consistent with these theories, individuals with medial temporal lobe amnesia can retrieve various types of remote knowledge, including the definitions of words, some world facts, and object names (Moscovitch et al., 2006; Verfaellie et al., 2000). However, recent research has revealed a few situations in which remote semantic memory retrieval is disrupted in individuals with medial temporal lobe amnesia (Klooster & Duff, 2015; Verfaellie et al., 2014; Race et al., 2013; Rosenbaum et al., 2009). In the present study, we asked whether these exceptions hint at a sustained role for the medial temporal lobes in the retrieval of experience-near knowledge, an episodic-like subcategory of semantic memory.
To address this question, we used a novel task designed to isolate remotely formed, dormant knowledge surrounding low-visibility occupations. Remotely formed, low-visibility occupations have many ideal qualities for studying the role of the medial temporal lobes in remote semantic memory. First, as confirmed by our self-report question, many middle-aged and older adults, including individuals with medial temporal lobe amnesia, are familiar with these occupations, which may reflect a combination of remote personal experience, media and literature exposure, and stories told to them. These occupations, because of their low visibility, are also rarely encountered in modern society, meaning most people have likely had few recent opportunities to update these concepts. The control participants' self-reported most recent exposure suggests this was the case in the current sample of individuals as well. We can therefore assume that probing knowledge of these sorts of occupations in our participants activated remote semantic memories that have been largely “dormant” for years. By extension, we can use remotely formed, low visibility occupations to ask whether the hippocampus/medial temporal lobes have a necessary role in remote semantic memory retrieval.
For experience-far semantic memory, the individuals with medial temporal lobe amnesia showed largely normal retrieval while describing remotely formed, low visibility occupations. The same general pattern was found for currently active, high visibility occupations as well. Overall, these results align with earlier research on vocabulary and object naming in medial temporal lobe amnesia (Moscovitch et al., 2006; Verfaellie et al., 2000) and prior work on autobiographical memory in amnesia (Wank et al., 2022; Grilli & Verfaellie, 2016). Taken together, the results appear to provide further support that the medial temporal lobes do not have a long-term role in the retrieval of many forms of abstract knowledge.
The results for experience-near semantic memory, however, lend themselves to a different interpretation. Here, we found a deficit in the retrieval of remote occupation-supporting experience-near semantic memories among the individuals with medial temporal lobe amnesia. The same pattern was evident for active occupations, indicating a profound loss of experience-near semantic memories. The individuals with bilateral hippocampal/medial temporal lobe lesions showed the greatest deficits in experience-near semantic memory. That said, only one of eight scores from the individuals with medial temporal lobe amnesia fell within the range of control scores (i.e., MTL3 generated eight remote experience-near semantic memories, whereas controls generated between seven and 15 remote experience-near semantic memories). This suggests that the amnesia-associated deficit in experience-near semantic memory, although most prominent in the face of bilateral hippocampal/medial temporal lobe lesions, is fairly robust. The findings from this novel semantic memory task, therefore, converge on the conclusion that lesions to the medial temporal lobe, especially bilateral lesions, may tend to selectively disrupt experience-near semantic memory, regardless of remoteness.
The experience-near semantic memory findings align with recent neuropsychological studies suggesting that the medial temporal lobes may have a sustained role in semantic memory under certain retrieval conditions, specifically when cognitive processes often associated with episodic memory are involved (Klooster & Duff, 2015; Verfaellie et al., 2014; Race et al., 2013; Rosenbaum et al., 2009). However, the cognitive processes underlying the connection between experience-near semantic memories and the hippocampus/medial temporal lobes remain an open question. Several theoretical models might be able to explain this brain–behavior relationship, including those suggesting that the hippocampus/medial temporal lobes have a necessary role in generative retrieval (Rosenbaum et al., 2009), relational processing (Duff et al., 2020; Konkel & Cohen, 2009), precision and spatiotemporal binding (Ekstrom & Yonelinas, 2020; Kolarik, Baer, Shahlaie, Yonelinas, & Ekstrom, 2018; Kolarik et al., 2016; Yonelinas, 2013), pattern separation (Yassa & Stark, 2011), and scene construction (Lynch et al., 2020; Maguire & Mullally, 2013; Hassabis, Kumaran, Vann, & Maguire, 2007). In fact, in comparison to experience-far semantic memories, experience-near semantic memories are arguably more specific and richer in detail because they have spatiotemporal content. As such, it is possible that the present findings reflect an extension of prior research showing that individuals with medial temporal lobe lesions have difficulty generating specific details of remotely learned stories (Verfaellie et al., 2014; Rosenbaum et al., 2009), rich features and specific names of concepts (Hilverman & Duff, 2021; Klooster & Duff, 2015), context-related features of concepts (Blumenthal et al., 2017), or specific details of repeated events involving a spatiotemporal context (Lynch et al., 2020; St-Laurent, Moscovitch, Levine, & McAndrews, 2009). Future research could attempt to systematically vary spatiotemporal content, specificity, and total detail/richness of experience-near semantic memory as this could provide clarity as to which mechanism(s) might be in play. Future research will also need to examine the role of retrieval difficulty on the apparent relationship between medial temporal lobe amnesia and semantic memory, as well as other forms of cognition (Yonelinas, 2013).
Regardless of the mechanism underlying the experience-near findings, these results appear to go along with recent theorizing against a strict episodic and semantic memory distinction (Andrews-Hanna & Grilli, 2021; Irish & Vatansever, 2020; Renoult et al., 2019; Grilli & Verfaellie, 2016). In other words, the findings reported here may be one more piece of evidence that some forms of semantic memory, in this case experience-near knowledge, share more cognitive and neural bases with episodic memory than they do with other forms of semantic memory, such as abstract, experience-far knowledge. By extension, however, these results appear to conflict with models of declarative memory that postulate semantic memories, once fully consolidated, are retrieved by the neocortex, and not the hippocampus/medial temporal lobes (Winocur & Moscovitch, 2011; Squire & Zola, 1998; Nadel & Moscovitch, 1997). Experience-near semantic memories, which remain partly attached to the spatiotemporal and event contexts of the experiences from which they were derived, may have a sustained reliance on the hippocampus/medial temporal lobes, as some models suggest is the case for episodic memory (Winocur & Moscovitch, 2011; Nadel & Moscovitch, 1997).
With human amnesia research, there often are caveats related to the lesions and neuropsychological profiles, and the present study is no exception. First, none of the individuals with medial temporal lobe amnesia had lesions isolated to the hippocampus. We therefore cannot conclude that remote experience-near semantic memories rely on the hippocampus specifically, as opposed to extrahippocampal medial temporal lobe structures. That said, as noted, the greatest deficits in experience-near semantic memories were found in the two individuals with bilateral, and thus the most extensive, hippocampal lesions. Second, all the individuals with medial temporal lobe amnesia had extramedial temporal lobe lesions. However, we think these extramedial temporal lobe lesions made little contribution to the results. This is because the cortical lesions were largely non-overlapping across the individuals with amnesia, but the pattern of reduced experience-near semantic memories was consistent among them. In addition, experience-far semantic memories were largely spared among the individuals with amnesia, suggesting that the cortical lesions had minimal impact on semantic memory. Third, there were nonsystematic deficits in attention/working memory, processing speed, and aspects of language across the individuals with medial temporal lobe amnesia. This was the case for the two bilateral medial temporal lobe participants, who had the greatest experience-near semantic memory deficits (numerically). That said, the semantic memory task was untimed, and participants were reminded of the key instructions after reach response. The task also did not require lengthy narrative responses or confrontation naming. It is also noteworthy that the individuals with amnesia were never confused by the task demands, nor did they forget the instructions or repeat themselves. The outcomes further held when we analyzed proportional data to even the playing field for language production. We therefore do not think these other cognitive deficits had much impact on the results, especially given that it is not clear why attention/working memory, processing speed, or language difficulties would selectively spare experience-far semantic memories. Nonetheless, a future study could provide clarity by examining individuals with isolated hippocampal and/or medial temporal lobe lesions who have selective episodic learning and memory impairment.
The occupation-defining semantic memory task that we used in the present study is novel and could be adapted in future research to address other questions. Although we required that participants were familiar with each occupation (and very familiar with most of them), it did turn out that, overall, remote occupations were less familiar to participants than the active occupations. This appears not to have had a significant impact on experience-near versus experience-far semantic memory generation, given that (1) there was not an effect of occupation type on overall memory generation, (2) occupation type did not interact with semantic memory subtype, and (3) the proportion of experience-near semantic memories provided for remote and active occupations did not vary. That said, a future study could systematically investigate the relationship between familiarity and experience-near versus experience-far semantic memory retrieval. A future study also could examine whether the recency of last exposure is associated with the generation of experience-near and experience-far semantic memories. This question would be better addressed by sampling from a much greater number of occupations and from more healthy adult participants. Finally, a future study could investigate whether similar outcomes arise when participants are required to generate a specified number of occupation-related semantic memories. The present study allowed participants to engage a natural stopping point, similar to our prior work on self-defining memories (Wank et al., 2022; Grilli & Verfaellie, 2016). There was a wide range of statements provided by control participants, which could reflect that we asked participants to generate defining features, as opposed to any relevant feature of the occupation. A modified task instruction could ensure a threshold is reached or that semantic fluency is an integrated feature of the study design.
To conclude, the present findings suggest that the hippocampus/medial temporal lobes may have a necessary and sustained role in the retrieval of experience-near semantic memory, an episodic-like subcategory of knowledge. On the one hand, such a conclusion adds a boundary condition to the idea that many forms of semantic memory are consolidated in the neocortex. On the other hand, the results are in line with theories that focus on the basic cognitive processes that may be critically supported by the hippocampus/medial temporal lobes (Ekstrom & Yonelinas, 2020; Kolarik et al., 2018; Yassa & Stark, 2011; Konkel & Cohen, 2009; Rosenbaum et al., 2009). What medial temporal-lobe-supported cognitive processes are essential to the representation of remotely or recently acquired experience-near semantic memories will need to be clarified by future studies. The present study, nonetheless, indicates that the hippocampus/medial temporal lobes may never relinquish control over the retrieval of knowledge that remains experience-near.
Acknowledgments
We would like to thank the research participants and their families for their time. We thank J. J. Bercel, Kiley Horn, Alex Novak, and Anna Robertson for assistance with data collection and scoring.
Reprint requests should be sent to Matthew Grilli, 1503 E. University Blvd., Tucson, AZ, 85721, or via e-mail: mdgrilli@arizona.edu.
Data Availability Statement
The scoring protocol and data are deposited here: https://osf.io/nas4f/?view_only=5dc87b011d6f4c7bb8c4ad7ddc1194e8.
Author Contributions
Matthew D. Grilli: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing—Original draft. Sameer Sabharwal-Siddiqi: Visualization; Writing—Review & editing. Sean C. Thayer: Data curation; Project administration; Writing—Review & editing. Steven Z. Rapcsak: Writing—Review & editing. Arne D. Ekstrom: Funding acquisition; Writing—Review & editing.
Funding Information
The authors acknowledge funding from the National Institute of Neurological Disorders and Stroke (NIH/NINDS; https://dx.doi.org/10.13039/100000065), grant number: R01NS114913 to A. D. E. and M. D. G.
Diversity in Citation Practices
Retrospective analysis of the citations in every article published in this journal from 2010 to 2021 reveals a persistent pattern of gender imbalance: Although the proportions of authorship teams (categorized by estimated gender identification of first author/last author) publishing in the Journal of Cognitive Neuroscience (JoCN) during this period were M(an)/M = .407, W(oman)/M = .32, M/W = .115, and W/W = .159, the comparable proportions for the articles that these authorship teams cited were M/M = .549, W/M = .257, M/W = .109, and W/W = .085 (Postle and Fulvio, JoCN, 34:1, pp. 1–3). Consequently, JoCN encourages all authors to consider gender balance explicitly when selecting which articles to cite and gives them the opportunity to report their article's gender citation balance. The authors of this paper report its proportions of citations by gender category to be: M/M = .417; W/M = .125; M/W = .146; W/W = .313.
REFERENCES
- Andrews-Hanna, J. R., & Grilli, M. D. (2021). Mapping the imaginative mind: Charting new paths forward. Current Directions in Psychological Science, 30, 82–89. 10.1177/0963721420980753, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley, A., Vargha-Khadem, F., & Mishkin, M. (2001). Preserved recognition in a case of developmental amnesia: Implications for the acquisition of semantic memory? Journal of Cognitive Neuroscience, 13, 357–369. 10.1162/08989290151137403, [DOI] [PubMed] [Google Scholar]
- Blumenthal, A., Duke, D., Bowles, B., Gilboa, A., Rosenbaum, R. S., Köhler, S., et al. (2017). Abnormal semantic knowledge in a case of developmental amnesia. Neuropsychologia, 102, 237–247. 10.1016/j.neuropsychologia.2017.06.018, [DOI] [PubMed] [Google Scholar]
- Conway, M. A. (2005). Memory and the self☆. Journal of Memory and Language, 53, 594–628. 10.1016/j.jml.2005.08.005 [DOI] [Google Scholar]
- Corkin, S. (2002). What's new with the amnesic patient H.M.? Nature Reviews Neuroscience, 3, 153–160. 10.1038/nrn726, [DOI] [PubMed] [Google Scholar]
- Craik, F. I. M. (2002). Levels of processing: Past, present...and future? Memory, 10, 305–318. 10.1080/09658210244000135, [DOI] [PubMed] [Google Scholar]
- Duff, M. C., Covington, N. V., Hilverman, C., & Cohen, N. J. (2020). Semantic memory and the hippocampus: Revisiting, reaffirming, and extending the reach of their critical relationship. Frontiers in Human Neuroscience, 13, 471. 10.3389/fnhum.2019.00471, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom, A. D., & Yonelinas, A. P. (2020). Precision, binding, and the hippocampus: Precisely what are we talking about? Neuropsychologia, 138, 107341. 10.1016/j.neuropsychologia.2020.107341, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elward, R. L., & Vargha-Khadem, F. (2018). Semantic memory in developmental amnesia. Neuroscience Letters, 680, 23–30. 10.1016/j.neulet.2018.04.040, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner, J. M., Brandt, K. R., Baddeley, A. D., Vargha-Khadem, F., & Mishkin, M. (2008). Charting the acquisition of semantic knowledge in a case of developmental amnesia. Neuropsychologia, 46, 2865–2868. 10.1016/j.neuropsychologia.2008.05.021, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli, M. D., & Verfaellie, M. (2014). Personal semantic memory: Insights from neuropsychological research on amnesia. Neuropsychologia, 61, 56–64. 10.1016/j.neuropsychologia.2014.06.012, [DOI] [PubMed] [Google Scholar]
- Grilli, M. D., & Verfaellie, M. (2016). Experience-near but not experience-far autobiographical facts depend on the medial temporal lobe for retrieval: Evidence from amnesia. Neuropsychologia, 81, 180–185. 10.1016/j.neuropsychologia.2015.12.023, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis, D., Kumaran, D., Vann, S. D., & Maguire, E. A. (2007). Patients with hippocampal amnesia cannot imagine new experiences. Proceedings of the National Academy of Sciences, U.S.A., 104, 1726–1731. 10.1073/pnas.0610561104, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilverman, C., Cook, S. W., & Duff, M. C. (2017). The influence of the hippocampus and declarative memory on word use: Patients with amnesia use less imageable words. Neuropsychologia, 106, 179–186. 10.1016/j.neuropsychologia.2017.09.028, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilverman, C., & Duff, M. C. (2021). Evidence of impaired naming in patients with hippocampal amnesia. Hippocampus, 31, 612–626. 10.1002/hipo.23325, [DOI] [PubMed] [Google Scholar]
- Irish, M., & Vatansever, D. (2020). Rethinking the episodic-semantic distinction from a gradient perspective. Current Opinion in Behavioral Sciences, 32, 43–49. 10.1016/j.cobeha.2020.01.016 [DOI] [Google Scholar]
- Klooster, N. B., & Duff, M. C. (2015). Remote semantic memory is impoverished in hippocampal amnesia. Neuropsychologia, 79, 42–52. 10.1016/j.neuropsychologia.2015.10.017, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klooster, N. B., Tranel, D., & Duff, M. C. (2020). The hippocampus and semantic memory over time. Brain and Language, 201, 104711. 10.1016/j.bandl.2019.104711, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolarik, B. S., Baer, T., Shahlaie, K., Yonelinas, A. P., & Ekstrom, A. D. (2018). Close but no cigar: Spatial precision deficits following medial temporal lobe lesions provide novel insight into theoretical models of navigation and memory. Hippocampus, 28, 31–41. 10.1002/hipo.22801, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolarik, B. S., Shahlaie, K., Hassan, A., Borders, A. A., Kaufman, K. C., Gurkoff, G., et al. (2016). Impairments in precision, rather than spatial strategy, characterize performance on the virtual Morris water maze: A case study. Neuropsychologia, 80, 90–101. 10.1016/j.neuropsychologia.2015.11.013, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel, A., & Cohen, N. J. (2009). Relational memory and the hippocampus: Representations and methods. Frontiers in Neuroscience, 3, 166–174. 10.3389/neuro.01.023.2009, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, K., Keane, M. M., & Verfaellie, M. (2020). The status of semantic memory in medial temporal lobe amnesia varies with demands on scene construction. Cortex, 131, 114–122. 10.1016/j.cortex.2020.07.005, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire, E. A., & Frith, C. J. (2003). Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain, 126, 1511–1523. 10.1093/brain/awg157, [DOI] [PubMed] [Google Scholar]
- Maguire, E. A., & Mullally, S. L. (2013). The hippocampus: A manifesto for change. Journal of Experimental Psychology: General, 142, 1180–1189. 10.1037/a0033650, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns, J. R., Hopkins, R. O., & Squire, L. R. (2003). Semantic memory and the human hippocampus. Neuron, 38, 127–133. 10.1016/S0896-6273(03)00146-6, [DOI] [PubMed] [Google Scholar]
- Martinelli, P., Sperduti, M., & Piolino, P. (2013). Neural substrates of the self-memory system: New insights from a meta-analysis. Human Brain Mapping, 34, 1515–1529. 10.1002/hbm.22008, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch, M., Nadel, L., Winocur, G., Gilboa, A., & Rosenbaum, R. S. (2006). The cognitive neuroscience of remote episodic, semantic and spatial memory. Current Opinion in Neurobiology, 16, 179–190. 10.1016/j.conb.2006.03.013, [DOI] [PubMed] [Google Scholar]
- Nadel, L., & Moscovitch, M. (1997). Memory consolidation, retrograde amnesia and the hippocampal complex. Current Opinion in Neurobiology, 7, 217–227. 10.1016/S0959-4388(97)80010-4, [DOI] [PubMed] [Google Scholar]
- Neisser, U. (1981). John Dean's memory: A case study. Cognition, 9, 1–22. 10.1016/0010-0277(81)90011-1, [DOI] [PubMed] [Google Scholar]
- O'Kane, G., Kensinger, E. A., & Corkin, S. (2004). Evidence for semantic learning in profound amnesia: An investigation with patient H.M. Hippocampus, 14, 417–425. 10.1002/hipo.20005, [DOI] [PubMed] [Google Scholar]
- Postle, B. R., & Corkin, S. (1998). Impaired word-stem completion priming but intact perceptual identification priming with novel words: Evidence from the amnesic patient H.M. Neuropsychologia, 36, 421–440. 10.1016/S0028-3932(97)00155-3, [DOI] [PubMed] [Google Scholar]
- Race, E., Carlisle, C., Tejwani, R., & Verfaellie, M. (2021). The language of mental images: Characterizing hippocampal contributions to imageable word use during event construction. Neuropsychologia, 151, 107705. 10.1016/j.neuropsychologia.2020.107705, [DOI] [PubMed] [Google Scholar]
- Race, E., Keane, M. M., & Verfaellie, M. (2013). Losing sight of the future: Impaired semantic prospection following medial temporal lobe lesions. Hippocampus, 23, 268–277. 10.1002/hipo.22084, [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2019). (https://www.R-project.org/). [Computer software]. [Google Scholar]
- Renoult, L., Davidson, P. S. R., Palombo, D. J., Moscovitch, M., & Levine, B. (2012). Personal semantics: At the crossroads of semantic and episodic memory. Trends in Cognitive Sciences, 16, 550–558. 10.1016/j.tics.2012.09.003, [DOI] [PubMed] [Google Scholar]
- Renoult, L., Davidson, P. S. R., Schmitz, E., Park, L., Campbell, K., Moscovitch, M., et al. (2015). Autobiographically significant concepts: More episodic than semantic in nature? An electrophysiological investigation of overlapping types of memory. Journal of Cognitive Neuroscience, 27, 57–72. 10.1162/jocn_a_00689, [DOI] [PubMed] [Google Scholar]
- Renoult, L., Irish, M., Moscovitch, M., & Rugg, M. D. (2019). From knowing to remembering: The semantic–episodic distinction. Trends in Cognitive Sciences, 23, 1041–1057. 10.1016/j.tics.2019.09.008, [DOI] [PubMed] [Google Scholar]
- Renoult, L., Tanguay, A., Beaudry, M., Tavakoli, P., Rabipour, S., Campbell, K., et al. (2016). Personal semantics: Is it distinct from episodic and semantic memory? An electrophysiological study of memory for autobiographical facts and repeated events in honor of Shlomo Bentin. Neuropsychologia, 83, 242–256. 10.1016/j.neuropsychologia.2015.08.013, [DOI] [PubMed] [Google Scholar]
- Rorden, C., & Brett, M. (2000). Stereotaxic display of brain lesions. Behavioural Neurology, 12, 191–200. 10.1155/2000/421719, [DOI] [PubMed] [Google Scholar]
- Rosenbaum, R. S., Gilboa, A., Levine, B., Winocur, G., & Moscovitch, M. (2009). Amnesia as an impairment of detail generation and binding: Evidence from personal, fictional, and semantic narratives in K.C. Neuropsychologia, 47, 2181–2187. 10.1016/j.neuropsychologia.2008.11.028, [DOI] [PubMed] [Google Scholar]
- Squire, L. R., & Zola, S. M. (1998). Episodic memory, semantic memory, and amnesia. Hippocampus, 8, 205–211. [DOI] [PubMed] [Google Scholar]
- St-Laurent, M., Moscovitch, M., Levine, B., & McAndrews, M. P. (2009). Determinants of autobiographical memory in patients with unilateral temporal lobe epilepsy or excisions☆. Neuropsychologia, 47, 2211–2221. 10.1016/j.neuropsychologia.2009.01.032, [DOI] [PubMed] [Google Scholar]
- Strikwerda-Brown, C., Mothakunnel, A., Hodges, J. R., Piguet, O., & Irish, M. (2019). External details revisited—A new taxonomy for coding ‘non-episodic’ content during autobiographical memory retrieval. Journal of Neuropsychology, 13, 371–397. 10.1111/jnp.12160, [DOI] [PubMed] [Google Scholar]
- Tanguay, A. N., Benton, L., Romio, L., Sievers, C., Davidson, P. S. R., & Renoult, L. (2018). The ERP correlates of self-knowledge: Are assessments of one's past, present, and future traits closer to semantic or episodic memory? Neuropsychologia, 110, 65–83. 10.1016/j.neuropsychologia.2017.10.024, [DOI] [PubMed] [Google Scholar]
- Teghil, A., Bonavita, A., Procida, F., Giove, F., & Boccia, M. (2022). Temporal organization of episodic and experience-near semantic autobiographical memories: Neural correlates and context-dependent connectivity. Journal of Cognitive Neuroscience, 34, 2256–2274. 10.1162/jocn_a_01906, [DOI] [PubMed] [Google Scholar]
- The Jamovi Project. (2023). jamovi (Version 2.3) [Computer Software]. Retrieved from https://www.jamovi.org. [Google Scholar]
- Tulving, E., Hayman, C. A., & Macdonald, C. A. (1991). Long-lasting perceptual priming and semantic learning in amnesia: A case experiment. Journal of Experimental Psychology: Learning, Memory, and Cognition, 17, 595–617. 10.1037/0278-7393.17.4.595, [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem, F., Gadian, D. G., Watkins, K. E., Connelly, A., Van Paesschen, W., & Mishkin, M. (1997). Differential effects of early hippocampal pathology on episodic and semantic memory. Science, 277, 376–380. 10.1126/science.277.5324.376, [DOI] [PubMed] [Google Scholar]
- Verfaellie, M., Bousquet, K., & Keane, M. M. (2014). Medial temporal and neocortical contributions to remote memory for semantic narratives: Evidence from amnesia. Neuropsychologia, 61, 105–112. 10.1016/j.neuropsychologia.2014.06.018, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaellie, M., Koseff, P., & Alexander, M. P. (2000). Acquisition of novel semantic information in amnesia: Effects of lesion location. Neuropsychologia, 38, 484–492. 10.1016/S0028-3932(99)00089-5, [DOI] [PubMed] [Google Scholar]
- Verfaellie, M., Reiss, L., & Roth, H. L. (1995). Knowledge of new English vocabulary in amnesia: An examination of premorbidly acquired semantic memory. Journal of the International Neuropsychological Society, 1, 443–453. 10.1017/S1355617700000540, [DOI] [PubMed] [Google Scholar]
- Wank, A. A., Robertson, A., Thayer, S. C., Verfaellie, M., Rapcsak, S. Z., & Grilli, M. D. (2022). Autobiographical memory unknown: Pervasive autobiographical memory loss encompassing personality trait knowledge in an individual with medial temporal lobe amnesia. Cortex, 147, 41–57. 10.1016/j.cortex.2021.11.013, [DOI] [PubMed] [Google Scholar]
- Winocur, G., & Moscovitch, M. (2011). Memory transformation and systems consolidation. Journal of the International Neuropsychological Society, 17, 766–780. 10.1017/S1355617711000683, [DOI] [PubMed] [Google Scholar]
- Yassa, M. A., & Stark, C. E. L. (2011). Pattern separation in the hippocampus. Trends in Neurosciences, 34, 515–525. 10.1016/j.tins.2011.06.006, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas, A. P. (2013). The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behavioural Brain Research, 254, 34–44. 10.1016/j.bbr.2013.05.030, [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The scoring protocol and data are deposited here: https://osf.io/nas4f/?view_only=5dc87b011d6f4c7bb8c4ad7ddc1194e8.